 |
|
|
|
|
|
|
| ||||||||||
|
|
|
|
|
|
|
||||
| ||||||||||
|
|
|
|
|
Persons using assistive technology might not be able to fully access information in this file. For assistance, please send e-mail to: mmwrq@cdc.gov. Type 508 Accommodation and the title of the report in the subject line of e-mail. Rubella Outbreak --- Arkansas, 1999Rubella is a viral disease that usually presents as a mild febrile rash illness in adults and children; however, 20%--50% of infected persons are asymptomatic. Rubella can have severe adverse effects on the fetuses of pregnant women who contract the disease during the first trimester of pregnancy, causing a wide range of congenital defects known as congenital rubella syndrome (CRS). The primary objective of the rubella vaccination program is to prevent intrauterine rubella infection. The primary strategies for rubella control in the United States are universal childhood vaccination, prenatal screening of pregnant women for rubella immunity, and vaccinating rubella-susceptible women postpartum. After the licensure of rubella vaccine in 1969, the incidence of rubella and CRS decreased 99% by 1997 (1). However, outbreaks continue to occur (2,3). During September 7--October 26, 1999, a total of 12 cases of rubella were confirmed in three Arkansas counties. This report describes this outbreak, which prompted reimplementation of routine rubella control and prevention measures. These included prenatal screening for rubella immunity and postnatal vaccination of rubella-susceptible women and the initiation of prevention and control activities in foreign-born populations that are less likely to be vaccinated. On September 7, a pregnant woman aged 23 years presented to a public health clinic in Fort Smith, Sebastian County, Arkansas, with rash and fever. The woman was from Mexico and had lived in Arkansas for 1 year before onset of illness. She later delivered a stillborn infant with pathologic findings compatible with intrauterine rubella infection. The index patient was a household contact of a Mexican aged 20 years who also was confirmed as infected with rubella by EIA testing. Both patients worked in a poultry processing plant in Fort Smith. Outbreak investigators interviewed household and workplace contacts, suspected patients, and potentially exposed pregnant women and tested them for rubella IgG and IgM antibodies. An additional 10 cases were confirmed by laboratory testing (Figure 1) in this and two other counties. A definitive laboratory diagnosis or epidemiologic link could not be established for an additional 14 patients (seven meeting the case definition for suspected and seven for probable rubella). Among the 12 confirmed cases, the median age was 23 years (range: 18--34 years); 10 (83%) were Hispanic, nine (75%) were foreign-born, and six (50%) were women. All six female patients were pregnant, and one became infected during the first trimester of pregnancy. Ten (83%) patients worked in poultry processing plants; the index patient and seven others worked at the same plant in Fort Smith. Nine of these 10 patients were Hispanic and were foreign-born (Mexico and El Salvador). Screening of pregnant women for rubella immunity was not part of routine prenatal care in Arkansas' public health clinics when this outbreak occurred. Because the index patient and other potential patients exposed persons in the clinic waiting room, and because the proportion of rubella-susceptible pregnant women attending the clinic was unknown, a serosusceptibility survey was conducted at the clinic during September 23--October 29. A questionnaire was administered to and serum specimens were taken from 155 women consecutively attending the clinic and tested for rubella IgG and IgM. Of the 155 women tested, 79 (51%) were Hispanic, 64 (41%) were white, five were black (3%), three (2%) were Asian, and four (3%) were of unknown race/ethnicity. Seventy-three (47%) women were foreign-born; 72 (99%) were born in Central America and Mexico. The median age was 23 years (range: 15--43 years). Of the 155 women, 46 (32%) reported a history of rubella vaccination, 25 (17%) had not been vaccinated, 74 (51%) did not know their rubella vaccination status, and no data were available for the remaining 10 (6%). In comparison with the relatively low number of women with a self-reported history of rubella vaccination, 134 (86%) women had positive test results for rubella IgG, 14 (9%) had negative test results, and seven (5%) had equivocal or missing test results. No association was found between IgG-positivity and nationality or history of vaccination. Of the 21 women who had equivocal or negative results, 11 (52%) reported a previous delivery in the United States, and 19 (90%) missed at least one opportunity for rubella vaccination. Reported by: P Dozier, J Bates, P Wiggins, J Wilhelm, Health Unit, Fort Smith; H Mabry, Northwest Arkansas Health Region; C Beets, J Burnett, M Foreman, L Gladden, L Himstedt, B Ledford, R Nugent, MD, K Sayyed, S Snow, MD, A Zoldessy, Arkansas Dept of Health. Div of Viral and Rickettsial Diseases, National Center for Infectious Diseases; Rubella/Mumps Activity, Div of Epidemiology and Surveillance, National Immunization Program; and an EIS Officer, CDC. Editorial Note:The findings in this report highlight the absence of routine, recommended prevention and control efforts in the state and the emergence of Hispanic, foreign-born persons as the main reservoirs of rubella virus in the United States. Prenatal screening followed by postpartum vaccination against rubella is essential for the control and elimination of CRS. Although recommended by the American College of Obstetricians and Gynecologists and the Advisory Committee on Immunization Practices (4), prenatal screening for rubella was discontinued in Arkansas public health clinics during the early 1980s because of fiscal constraints. In the absence of routine prenatal screening for rubella antibodies, the immune status of pregnant women potentially exposed to rubella virus was unknown. In the United States, prenatal screening and postpartum vaccination might prevent an estimated 50% of all CRS cases (5). Based on supplementary data reported through the national notifiable diseases surveillance system in the United States, rubella primarily affects foreign-born Hispanic adults. Among rubella patients with known ethnicity in the United States, the proportion of Hispanics increased from 19% in 1992 to 79% in 1998, compared with 83% of patients in this outbreak. In the affected plant in Fort Smith, a large proportion of the workforce was Hispanic, and many of these were born and raised abroad. In Latin America, many countries have only recently introduced rubella into their routine childhood vaccination programs. For immigrants entering the United States, vaccination efforts focus on preschool-aged children and students; adults are not routinely screened or vaccinated. To eliminate rubella and CRS in the United States, further control efforts are needed to identify and vaccinate clusters of rubella-susceptible adults and to ensure nationwide prenatal rubella screening and postpartum vaccination of rubella-susceptible women. As a result of this outbreak, the Arkansas Department of Health (ADH), in collaboration with employers, implemented additional control efforts that focused on workplace vaccination. ADH implemented a measles-mumps-rubella (MMR) vaccine screening policy at a local employment agency that supplied temporary help for the poultry processing companies. Potential employees were required to show proof of a previous MMR vaccination or receive MMR vaccine before employment. In addition, ADH recommended that employers of large numbers of foreign-born persons provide vaccine at the plant site and offered clinics to any industry that employed large numbers of foreign-born persons in Arkansas. ADH has reimplemented routine screening for rubella immunity in all maternity and family planning clinics. Susceptible ADH maternity patients are identified routinely and offered MMR vaccine postpartum, and family planning patients are offered MMR vaccine immediately with appropriate counseling. These measures have resulted in substantial increases in rubella seropositivity rates for pregnant women in ADH clinics. Control efforts such as these in conjunction with proven routine measures are necessary to eliminate indigenous rubella and CRS in the United States. References
Figure 1 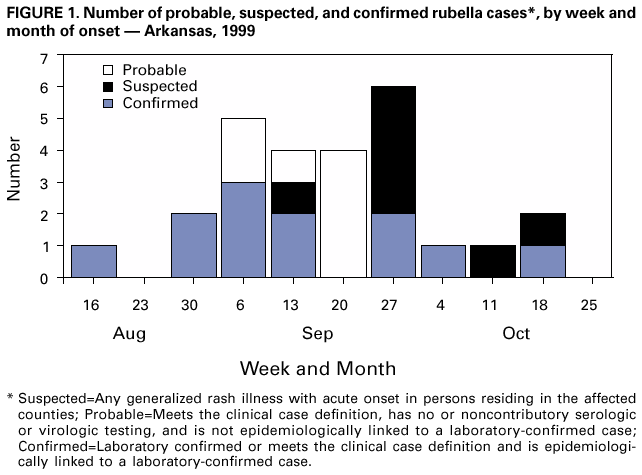 Return to top.
Disclaimer All MMWR HTML versions of articles are electronic conversions from ASCII text into HTML. This conversion may have resulted in character translation or format errors in the HTML version. Users should not rely on this HTML document, but are referred to the electronic PDF version and/or the original MMWR paper copy for the official text, figures, and tables. An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S. Government Printing Office (GPO), Washington, DC 20402-9371; telephone: (202) 512-1800. Contact GPO for current prices. **Questions or messages regarding errors in formatting should be addressed to mmwrq@cdc.gov.Page converted: 12/20/2001 |
|||||||||
This page last reviewed 12/20/2001
|