 |
|
|
|
|
|
|
| ||||||||||
|
|
|
|
|
|
|
||||
| ||||||||||
|
|
|
|
|
Persons using assistive technology might not be able to fully access information in this file. For assistance, please send e-mail to: mmwrq@cdc.gov. Type 508 Accommodation and the title of the report in the subject line of e-mail. Global Progress Toward Laboratory Containment of Wild Polioviruses, June 2001When the World Health Assembly resolved to eradicate poliomyelitis in 1988, the estimated number of polio cases was 350,000; in 2000, approximately 3000 cases were reported (1). Two World Health Organization (WHO) regions (the Americas and Western Pacific) have been certified as polio-free, and a third (European Region) has been free of indigenous wild poliovirus transmission for nearly 3 years (3 years are required for WHO certification). As interruption of wild poliovirus circulation approaches, public health agencies are increasing efforts to minimize the risk for reintroduction of wild polioviruses from laboratory sources. This report describes the global plan for containing laboratory wild polioviruses and summarizes the steps being taken toward implementation. Once wild poliovirus transmission ceases and laboratories are the only source of wild poliovirus, an increase in precautions will be needed to minimize the risk for reintroducing wild polioviruses from stored sources and for ensuring the safe handling and disposal of these materials, which include wild poliovirus infectious stocks, specimens from polio patients, and products of research or potentially infectious materials (i.e., throat, fecal, or environmental [water and sewage] specimens collected for any purpose at a time and in a geographic location where polio was endemic). Virology laboratories are the most likely sources of infectious materials, but other biomedical laboratories such as bacteriology, parasitology, gastroenterology, nutrition, pathology, and environmental also may store infectious materials. The WHO Global Action Plan for Laboratory Containment of Wild Polioviruses (2), developed in collaboration with scientists, ministries of health, and vaccine manufacturers, was endorsed by a 1999 World Health Assembly resolution. The Global Certification Commission stated that a precondition of certification is adequate containment of wild polioviruses (3), and the plan outlines three implementation phases: preglobal eradication, postglobal eradication, and post-OPV (oral poliovirus vaccine) immunization. During the preglobal eradication phase, countries in which wild poliovirus circulation has been interrupted appoint a national task force or coordinator to develop and oversee a national plan. The first step in the plan is to alert biomedical laboratories to the impending eradication of polio, encourage them to dispose appropriately of unneeded wild polio-virus or potentially infectious materials, and establish a national inventory of laboratories that retain such materials. The inventory will provide a list of laboratories to be informed of eradication progress and containment developments and to be notified when eradication occurs and implementation of additional biosafety requirements takes effect. Many countries/territories are surveying and identifying laboratories for their capacity to store infectious materials (Table 1). Laboratories that do not have the capacity to store infectious materials or routinely do not keep specimens for long periods confirm their inability to serve as a storage facility and are eliminated as a site for wild poliovirus materials. Laboratories identified as having the capacity to store infectious materials are followed up to determine the materials they hold in storage. The postglobal eradication phase begins soon after detection of the last wild poliovirus in the world. At that time, laboratories storing and handling infectious or potentially infectious materials prepare for certification by implementing biosafety conditions appropriate for the levels of risk presented by the materials under study and laboratory procedures in use. A further increase in biosafety requirements is anticipated when a global decision is made on OPV cessation. WHO is working with manufacturers of inactive polio vaccine (IPV) to develop a plan for containing poliovirus strains used in manufacturing IPV and to formulate containment guidelines designed to minimize risk during the production of IPV. The risk for accidental reintroduction of wild poliovirus into a community from a laboratory is possible if four conditions exist: 1) the presence of wild poliovirus infectious materials in a laboratory; 2) an event (e.g., break in standard procedure) that exposes workers to infectious materials containing poliovirus; 3) susceptible workers who replicate and shed the virus in their stool; and 4) susceptible persons in the community who are directly or indirectly exposed to an infected worker. Implementation of the plan cannot ensure absolute containment; however, it will minimize the likelihood of a situation in which the first three conditions occur. The fourth condition is linked to posteradication immunization policy decisions. Progress is being made in implementing the first phase of laboratory containment (Table 1); 110 (51%) of 216 countries/territories have appointed a national task force and have created a plan. Eleven countries have submitted completed national inventories, and approximately 400 laboratories with wild poliovirus materials have been identified. In the Americas, laboratory containment activities are under way. Canada is in the final stages of preparing its national inventory and the United States is in the initial stage of its laboratory survey. In the Western Pacific, all member states have begun implementation and nine of 36 have finished their national inventory. Laboratory containment activities have increased substantially in the European Region as it prepares for certification; 48 of 51 member states have appointed a task force and 36 of these have started contacting laboratories. Although polio is still endemic in the South-East Asian, Eastern Mediterranean, and African regions, many polio-free countries in these regions have begun preparations for laboratory containment. Reported by: Vaccines and Biologicals Dept, World Health Organization, Geneva, Switzerland. Respiratory and Enteric Viruses Br, Div of Viral and Rickettsial Diseases, National Center for Infectious Diseases; Vaccine Preventable Disease Eradication Div, National Immunization Program, CDC. Editorial Note:Appropriate laboratory containment of wild poliovirus is critical to polio eradication. Progress toward implementation of the global plan is encouraging; a systematic and well-documented approach has been established to identify laboratories with infectious wild poliovirus or potentially infectious materials, and cooperation from laboratories and governments has been good throughout the world. Implementing laboratory containment procedures is a complex process. Industrialized countries with well-developed research programs and laboratory infrastructure will require considerable time and effort for implementing survey and inventory activities. Countries with less developed biomedical research programs and laboratory infrastructure generally do not have laboratories that store infectious materials. Such countries can more easily compile a list of laboratories and identify those with infectious wild poliovirus or potentially infectious materials. Technical expertise for assisting countries with their national plans and implementing activities is available from members of the Global Laboratory Network for Polio Eradication, which comprises 124 national (or subnational) laboratories, 16 regional reference laboratories, and seven specialized laboratories. The link between certification and laboratory containment activities has evolved; laboratory containment procedures were not part of the certification process when the Americas was certified free of polio in 1994. The Pan American Health Organization is working with member governments to meet the requirements outlined in the global plan. The most progress toward completion of the first phase of the plan has been reported from the Western Pacific Region where laboratory containment activities were an integral part of the certification process. The European Region is integrating containment into the regional certification process. WHO member states will be responsible for laboratory containment within their respective countries. The containment process will be monitored by national authorities, national committees for polio eradication, and the Regional and Global Certification commissions. Before global certification can occur, as anticipated in 2005, all countries of the world must demonstrate that they have minimized the risk for reintroducing wild polio-virus from their laboratories to a polio-free world. References
Table 1 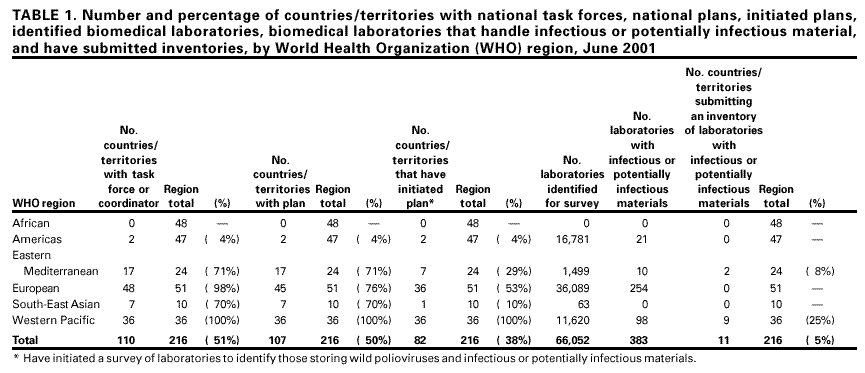 Return to top. Disclaimer All MMWR HTML versions of articles are electronic conversions from ASCII text into HTML. This conversion may have resulted in character translation or format errors in the HTML version. Users should not rely on this HTML document, but are referred to the electronic PDF version and/or the original MMWR paper copy for the official text, figures, and tables. An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S. Government Printing Office (GPO), Washington, DC 20402-9371; telephone: (202) 512-1800. Contact GPO for current prices. **Questions or messages regarding errors in formatting should be addressed to mmwrq@cdc.gov.Page converted: 7/26/2001 |
|||||||||
This page last reviewed 7/26/2001
|