 |
|
|
|
|
|
|
| ||||||||||
|
|
|
|
|
|
|
||||
| ||||||||||
|
|
|
|
|
Persons using assistive technology might not be able to fully access information in this file. For assistance, please send e-mail to: mmwrq@cdc.gov. Type 508 Accommodation and the title of the report in the subject line of e-mail. Update: Outbreak of Rift Valley Fever --- Saudi Arabia, August--November 2000On September 10, 2000, the Ministry of Health (MOH), Kingdom of Saudi Arabia and subsequently, the MOH of Yemen began receiving reports of unexplained hemorrhagic fever in humans and associated animal deaths and abortions from the far western Saudi-Yemeni border region. These cases subsequently were confirmed as Rift Valley fever (RVF), the first such cases on the Arabian peninsula (1). This report updates the findings of the ongoing investigation conducted by the Saudi Arabian MOH in collaboration with CDC and the National Institute of Virology, South Africa. As of November 1 in Saudi Arabia, 516 persons with suspected severe RVF* requiring hospitalization have been reported from primary health-care centers and hospitals (Figure 1); 87 (17%) have died. Suspected cases have been identified through an elaborate pre-existing system of primary health-care centers that refer acutely ill persons to district hospitals for assessment of hepatitis and other criteria for admission as RVF case-patients. Of the 216 suspected severe case-patients with appropriate serum samples, 206 (95%) have been laboratory confirmed by either viral antigen or IgM antibody testing. Of the 516 case-patients, 407 (79%) were male; the median age was 46 years (range: 1--95 years); the youngest confirmed patient was aged 14 years; and 424 (82%) were Saudi citizens, 80 (16%) were Yemeni citizens, and 12 (2%) were of other nationalities. The largest number of cases have been reported from the southwestern province of Jazan (365 [77%]), and 122 (24%) cases have been reported from the contiguous Asir region. Except for one case-patient in Al Quenfadah, northwest of Jazan, all other case-patients had traveled recently to Jazan or Asir. The mean duration from disease onset to hospitalization was 3.3 days (standard deviation [SD]= ±3.2 days), and the average time from disease onset to death among the 87 fatalities was 6.3 days (SD= ±5.3 days). Of 148 case-patients at King Fahad Central Hospital in Jazan, 57 (39%) with mild to moderate RVF disease had reversible acute renal failure, requiring only supportive care for 2--14 days; 27 (18%) with severe disease required hemodialysis. Based on preliminary data from the ongoing epidemiologic investigation, 125 (76%) of 165 case-patients reported close contact with animals, especially sheep and goats, and 91 (64%) of 143 case-patients reported a history of exposure to dead, and/or aborted animals. Nearly all persons reported having had mosquito bites and that the mosquitoes were present at their place of residence. Entomologic studies found large numbers of two species of mosquitoes, Culex tritaeniorrhynchus and Aedes caspius, in the flood irrigation farming areas at the foot of the mountains and the foothills of Al Ardah district in Jazan, where the first and most human cases were reported. Preliminary laboratory studies have already yielded isolates of RVF virus from both of these species. Further laboratory identification of the collected mosquitoes suggests the presence of additional Aedes species; definitive species typing is pending. A regional survey for RVF antibody prevalence in domestic ungulates, primarily goats and sheep, was conducted in Jazan and Asir provinces. RVF anti body prevalence >90% was found in Al Ardah district. RVF antibodies also were found among ungulates in other surveyed areas. A correlation was found between areas where human cases were reported and the same flood irrigation farming areas in the upper reaches of the wadis identified by the entomologists. Reported by: H Arishi, MD, A Ageel, MD, M Abdu Rahman, MD, A Al Hazmi, MD, AR Arishi, MD, B Ayoola, MD, C Menon, MD, J Ashraf, MD, O Frogusin, MD, L Ochia, F Sawwan, M Al Hazmi, MD, Medical Svcs, King Fahad Central Hospital, Jazan; M Almaradni, MD, Medical Svcs, Al Ardah Hospital, Jazan; M Yasim Shah, MD, Medical Svcs, Samta General Hospital, Jazan; A As-Sharif, MS, M Al Sayed, Preventive Medicine, A Raheem Ageel, MSD, Regional Health Affairs, Jazan; A Shihry, MD, Al Khobar, Eastern Province; A Abudahish, PharmD, A Al Sharif, MD, Abha, Asir Province; I Al Hazmi, Al Quenfadah; An A Alrajhi, MD, King Faisal Specialist Hospital and Research Center, Riyadh; MA Al-Hedaithy, MD, College of Medicine, King Khalid Univ Hospital, Riyadh; A Fatani, MD, A Sahaly, MD, A Ghelani, MD, T Al Basam, MD, A Turkistani, DDS, AM Al Rabeah, N Al Hamdan, MD, Saudi Arabia Field Epidemiology Training Program, Riyadh; A Mishkas, MBBS, Infectious Diseases; MH Al Jeffri, MD, Parasitic and Infectious Diseases; YY Al Mazrou, MD, M MA Alamri, MM Al-Qahtani, MD, A Al Drees, Laboratories and Blood Banks, Riyadh; T Madani, MD, G Al Gasabi, MD, OA Shubokshi, MD, Ministry of Health, Saudi Arabia; M Al Khamees, DVM, D Al Mujalli, DVM, A Aziz Ibn Moamar, PhD, Ministry of Agriculture and Water, Riyadh, Saudi Arabia. World Health Organization, Geneva, Switzerland. P Jupp, PhD, A Kemp, MS, F Burt, PhD, R Swanepoel, PhD, Special Pathogens Unit, National Institute of Virology, Johannesburg, South Africa. Infectious Disease Pathology Activity, Special Pathogens Br, Div of Viral and Rickettsial Diseases, National Center for Infectious Diseases; and an EIS Officer, CDC. Editorial Note:RVF is a mosquito-borne zoonotic disease affecting domestic ungulates (especially goats and sheep) characterized by large epizootics during periods of heavy rainfall with associated outbreaks in humans. Most human infection is associated with an uncomplicated febrile illness or is inapparent. More severe complications include retinitis, hepatitis, renal failure, hemorrhagic fever, encephalitis, and death. This outbreak extends the geographic distribution of known infection outside of Africa and indicates this virus may be able to establish itself almost anywhere in the world based on the availability of potential permissive vectors and animal reservoirs. Official reports from Yemen suggest ongoing transmission over a large area, compared with the outbreak in Saudi Arabia, which is more circumscribed and is now mainly focused in Asir province. However, the differing case definitions and surveillance methodologies preclude a direct comparison of the Saudi Arabian and Yemeni outbreaks. Nevertheless, these outbreaks demonstrate disease transmission in an approximately 600 km area, including the flood plains of the wadis extending from the Sarawat mountains to the Red Sea coastal plain and extending from the Hodediah governate in Yemen to the Al Quendafah health region in Saudi Arabia. Epidemiologic data suggest the simultaneous, extensive, and multicentric nature of the outbreaks rather than radiation of disease from a single focus in Saudi Arabia or Yemen. Control and prevention measures are ongoing in these countries as are preparations for studies to better define risk factors for infection and severe disease, examine the risk for nosocomial infection, gauge the magnitude and scope of the outbreak, characterize viral sequences from isolates, test the efficacy of intravenous ribavirin, and determine the prevalence of infection among captured vector species. The abundance of A. caspius (a floodwater breeding aedine mosquito) breeding in the flooded agricultural fields suggests that this species can act as an interepidemic (reservoir) host for the virus and an epidemic vector when heavy rains promote mosquito population explosions; C. tritaeniorrhynchus is probably an epidemic vector. Continued surveillance will be necessary to determine if these infected "floodwater" Aedes, the major vector for persistence of the virus in Africa attributed to transovarial transmission, supports establishment of RVF on the Arabian Peninsula. Reference
* Suspected severe RVF is defined as unexplained illness >48 hours in duration associated with threefold elevation in transaminases (alanine aminotransferase, aspartate aminotransferase, and gamma glutamyl transpeptidase) or clinical jaundice; or unexplained illness >48 hours in duration associated with abortion or bleeding manifestations (e.g., from puncture sites, ecchymosis, petechiae, purpura, epistaxis, gastrointestinal bleeding, or menorrhagia); or unexplained illness >48 hours in duration associated with neurologic manifestations (e.g., vertigo, confusion, disorientation, amnesia, lethargy, hallucination, meningismus, choreiform movements, ataxia, tremor, convulsions, hemiparesis, decerebrate posturing, locked-in syndrome, or coma); or unexplained illness >48 hours in duration associated with fever, diarrhea, nausea, vomiting, or abdominal pain and any one of the following laboratory values: 1) hemoglobin <8 gm/dL; 2) platelets <100,000 mm3 (<10 x 1010/L); 3) LDH 2 x upper limit of normal; 4) creatinine >150 mol/L; or 5) CPK 2 x upper limit of normal; or unexplained death with recent history of fever during the preceding 2 weeks; and if a specimen is available, evidence of RVF-specific antigen or IgM antibody. Specimens must be obtained at least 7 days after illness onset before they can be considered negative. Figure 1 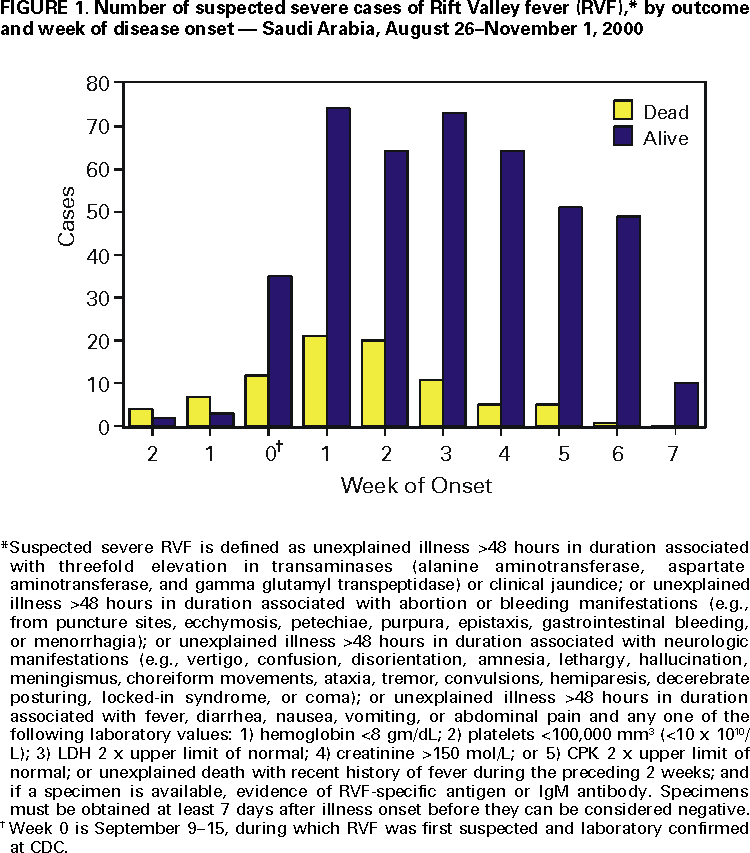 Return to top. Disclaimer All MMWR HTML versions of articles are electronic conversions from ASCII text into HTML. This conversion may have resulted in character translation or format errors in the HTML version. Users should not rely on this HTML document, but are referred to the electronic PDF version and/or the original MMWR paper copy for the official text, figures, and tables. An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S. Government Printing Office (GPO), Washington, DC 20402-9371; telephone: (202) 512-1800. Contact GPO for current prices. **Questions or messages regarding errors in formatting should be addressed to mmwrq@cdc.gov.Page converted: 11/2/2000 |
|||||||||
This page last reviewed 5/2/01
|