 |
|
|
|
|
|
|
| ||||||||||
|
|
|
|
|
|
|
||||
| ||||||||||
|
|
|
|
|
Persons using assistive technology might not be able to fully access information in this file. For assistance, please send e-mail to: mmwrq@cdc.gov. Type 508 Accommodation and the title of the report in the subject line of e-mail. Folate Status in Women of Childbearing Age --- United States, 1999In 1992, the U.S. Public Health Service (PHS) recommended that women of child-bearing age increase consumption of the vitamin folic acid to reduce spina bifida and anencephaly (neural tube defects [NTD]) cases (1). Since then, national efforts have been implemented to increase the use of dietary supplements containing folic acid (2). In 1996, the U.S. Food and Drug Administration (FDA) mandated that all enriched cereal grain products be fortified with folic acid (3). To assess levels of folic acid among childbearing-aged women, CDC compared serum and red blood cell (RBC) folate concentrations for childbearing-aged women who participated in the 1999 National Health and Nutrition Examination Survey (NHANES 1999) to childbearing-aged women who participated in the Third National Health and Nutrition Examination Survey (NHANES III, 1988--1994). The findings indicate substantial increases in serum and RBC folate concentrations among women of childbearing age. Both NHANES III and NHANES 1999 used a stratified, multistage probability sample of the civilian, U.S. noninstitutionalized population. NHANES III surveyed persons aged >2 months. NHANES 1999 surveyed persons of all ages. A household interview and a physical examination were conducted for each survey participant. During the physical examination, blood was collected by venipuncture for all persons aged >1 year. Serum and RBC folate were measured by the same analyst in the NHANES Central Laboratory for both NHANES III and NHANES 1999. For Phase 2 (1991--1994) of NHANES III and for NHANES 1999, the Bio-Rad Quantaphase II™ simultaneous folate/vitamin B12 radioassay (Bio-Rad Laboratories, Hercules, California) was used; the Quantaphase™ assay (folate alone) was used for Phase 1 of NHANES III (1988--1991) (4). Longterm quality-control data for these assays, including "bridge" control materials that were used in both surveys, indicated no analytical drift; results of all external proficiency testing challenges were graded as satisfactory. The overall >6 year mean coefficient of variation for serum and RBC folate was 5%. From NHANES III to NHANES 1999, mean serum folate concentrations for all women aged 15--44 years increased from 6.3 to 16.2 ng/mL, and the 75th percentile increased from 7.8 to 19.5 ng/mL (Table 1). Increases in the mean serum folate concentration were of comparable magnitude for nonpregnant women (6.0 to 15.9 ng/mL), a group less likely to use folic acid-containing supplements, for women who had used a vitamin/mineral supplement at least once during the preceding 30 days (8.4 to 20.0 ng/mL), and for women who had not used supplements (4.7 to 12.6 ng/mL). Similar results were obtained for RBC folate, a better measure of longterm folate status. Mean RBC folate concentrations for all women aged 15--44 years increased from 181 to 315 ng/mL (Table 1). Reported by: National Center for Health Statistics, National Center for Chronic Disease Prevention and Health Promotion, and National Center for Environmental Health, CDC. Editorial Note:Results from NHANES 1999, which was conducted after implementation of food fortification and educational efforts to increase folate consumption, suggest that these public health actions have been effective in increasing folate status among U.S. women of childbearing age. These findings are consistent with reports of improved folate status in selected subsets of the U.S. population (5,6). One of the national health objectives for 2010 is to increase the proportion of pregnancies begun with an optimum folic acid level by increasing the median RBC folate level to 220 ng/mL among nonpregnant women aged 15--44 years (objective 16-6b) (7). On the basis of NHANES 1999, this objective has been met. Women of childbearing age in the United States who are capable of becoming pregnant should consume 0.4 mg of folic acid per day to reduce their risk for having a pregnancy affected with spina bifida or other NTDs (1). The use of vitamin supplements containing folic acid before and during early pregnancy reduces the risk for NTD (1). In addition, PHS recommended and FDA subsequently mandated fortification of the food supply to deliver folic acid to the general population. Because up to half of pregnancies are unplanned and NTDs occur early in pregnancy, before many women are aware that they are pregnant, food fortification is a particularly important approach to folic acid delivery. The increase in blood folate levels among women of childbearing age participating in NHANES 1999 is probably the result of the fortification of enriched cereal grain products, although some of the increase may be attributable to educational efforts and an increase in women using vitamin supplements containing folic acid. Preliminary analyses indicate that the prevalence of supplement use was similar in the two surveys. Other studies have documented relatively small increases in the proportion of childbearing-aged women who regularly consume supplements containing folic acid (8,9). In addition, blood folate concentrations in women who did not use vitamin supplements also were higher in NHANES 1999 than in NHANES III. Because the sample size in NHANES 1999 is smaller than that of the multiyear NHANES III, more data will be necessary to confirm these findings and to allow more detailed analyses of trends in biochemical folate status in all population subgroups, particularly in young women of different race/ethnicity and socioeconomic status. If all women of childbearing age followed the PHS recommendation of daily folic acid consumption, the number of pregnancies affected by NTD would be reduced by half (1). Despite the substantial increase in blood folate concentrations documented for U.S. women of childbearing age, full evaluation of the health impact of folic acid fortification on NTD occurrence will require additional information. Data on NTD occurrence derived from the birth certificates of babies born in 1999 (conceived in 1998, after fortification became mandatory) are scheduled to be released in 2000. These national data, along with other NTD data collected by CDC and additional analyses of data from the continuous and ongoing NHANES, will provide data to evaluate fully the impact of folic acid fortification in the United States. References
Table 1 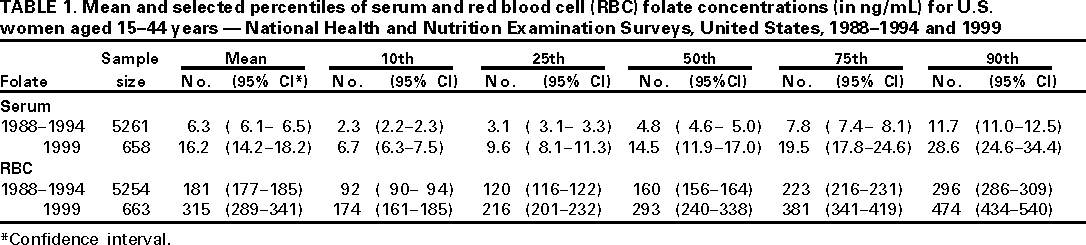 Return to top. Disclaimer All MMWR HTML versions of articles are electronic conversions from ASCII text into HTML. This conversion may have resulted in character translation or format errors in the HTML version. Users should not rely on this HTML document, but are referred to the electronic PDF version and/or the original MMWR paper copy for the official text, figures, and tables. An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S. Government Printing Office (GPO), Washington, DC 20402-9371; telephone: (202) 512-1800. Contact GPO for current prices. **Questions or messages regarding errors in formatting should be addressed to mmwrq@cdc.gov.Page converted: 10/26/2000 |
|||||||||
This page last reviewed 5/2/01
|