 |
|
|
|
|
|
|
| ||||||||||
|
|
|
|
|
|
|
||||
| ||||||||||
|
|
|
|
|
Persons using assistive technology might not be able to fully access information in this file. For assistance, please send e-mail to: mmwrq@cdc.gov. Type 508 Accommodation and the title of the report in the subject line of e-mail. Enterovirus Surveillance --- United States, 1997--1999Enteroviruses account for an estimated 10--15 million symptomatic infections in the United States each year (1). At present, 66 serotypes of enteroviruses are recognized, including three poliovirus serotypes (2). A range of diseases is associated with nonpolio enterovirus infections, including aseptic meningitis, encephalitis, neonatal enteroviral disease, myocarditis, pericarditis, chronic infections among persons with compromised immune systems, poliomyelitis-like illness, hand-foot-and-mouth disease, nonspecific upper respiratory disease, and other manifestations (3). This report summarizes data from the National Enterovirus Surveillance System (NESS) and describes temporal trends of reported enterovirus infections in the United States during 1997--1999. From January 1997 through December 1999, state public health laboratories reported to CDC 1741 enterovirus isolates, including 1672 isolates of nonpolio enteroviruses (Table 1) and 69 isolates of vaccine-related polioviruses. The number of states reporting enterovirus isolations declined from 14 in 1997 to eight in 1999. Of the 1672 nonpolio enterovirus isolates, echovirus 30 was the predominant serotype and accounted for 27.5% of all isolates, followed by echovirus 11 (13.8%), echovirus 9 (8.7%), and echovirus 6 (6.9%). Enterovirus serotype was reported as unknown for 13.1% of the isolates. The 15 most common serotypes accounted for 88.6%--98.2% of all isolates each year. Of the 63 known nonpolio enterovirus serotypes, 38 were reported during 1997--1999. Of these, 15 serotypes (coxsackie viruses A9, B2, B3, B4, B5; echoviruses 4, 5, 6, 9, 11, 16, 18, 25, 30; and enterovirus 71) have been reported in each of the 3 years. Twelve of these serotypes were among the 15 most common enteroviruses reported during 1997--1999. During 1997--1999, the proportion of isolates for some serotypes, such as echoviruses 6, 7, 11, and 30, varied widely, and the proportion of isolates for some other serotypes (e.g., coxsackieviruses B2 and B4) remained relatively low but constant. In addition to nonpolio enteroviruses, 69 isolates of vaccine-related polioviruses were reported (3.9% of all enterovirus isolates). The number of vaccine-related poliovirus isolates declined from 47 (8.2%) in 1997 to 19 (2.3%) in 1998, to three (0.8%) in 1999. Of the 25.3% of reports that included clinical information, most of the reported diagnoses were aseptic meningitis (37.6%) or respiratory illness (9.3%) and a smaller percentage were encephalitis (4.1%) and carditis and paralytic illness (0.2%). The source for enterovirus isolation was the cerebrospinal fluid (44.2% of reports), a stool specimen or a rectal swab (24.2%), a nasopharyngeal specimen (20.9%), and a urine sample (1.1%). For 9.6% of reports, the source of enterovirus isolation was not noted. Children aged <1 year accounted for 45% of all reported enterovirus isolates. Reported by: State virology laboratory directors. Respiratory and Enteric Viruses Br, Div of Viral and Rickettsial Diseases, National Center for Infectious Diseases, CDC. Editorial Note:To monitor temporal patterns of enterovirus circulation, state public health laboratories voluntarily report enterovirus isolates by serotype to CDC through NESS. The findings in this report are consistent with previous observations on temporal variability of predominant serotypes. Some serotypes appear to circulate endemically and others circulate in a cyclical fashion with epidemic years followed by years with decreased activity (1). Of the 15 most common serotypes during 1997--1999, 10 serotypes (echoviruses 30, 11, 9, 6, and 7; coxsackieviruses B2, A9, B3, and B4; and enterovirus 71) were among the most common enteroviruses during 1993--1996 (4). Of these, only enterovirus 71 was not included among the predominating serotypes during 1970--1983 (1). The proportion of less common serotypes declined from 17.8% during 1993--1996 (4) to 6.3% during 1997--1999. The proportion of enterovirus isolates of unknown serotype increased from 3.8% of all isolates during 1993--1996 (4) to 13.1% during 1997--1999. The decline in numbers of vaccine-related poliovirus isolates during 1997--1999 probably resulted from declining use of oral polio vaccine (OPV) in the United States. To prevent cases of vaccine-associated polio, CDC's Advisory Committee on Immunization Practices recommended transition from an all-OPV schedule to a sequential schedule of polio vaccination (i.e., two doses of inactivated polio vaccine followed by two doses of OPV) beginning in 1997 (5) with further narrowing of the options for administering OPV beginning in 1999 (6). Enterovirus surveillance data provide information for detecting major temporal trends in enterovirus circulation in the United States. However, the data may not be representative of the general U.S. population because of the limited number of reporting laboratories. In addition, this number has declined from 25 in 1993, to 14 in 1996 (4), to eight in 1999. This decline is of concern, especially at a time when enterovirus antiviral drugs are being developed (7,8). Because of the variability in susceptibility of different enterovirus serotypes to some antiviral drugs (9), data about the circulating serotypes will be helpful in considering the impact of these drugs on enterovirus disease. Enterovirus surveillance data also are important for use in confirming that wild poliovirus has been eradicated from the United States. Finally, new methods, such as the polymerase chain reaction assay and sequencing studies, are improving the ability to diagnose and serotype enterovirus infections (2,10) and may improve surveillance for enterovirus serotypes. CDC is considering changes to promote more complete and timely reporting of enterovirus surveillance data and to include new approaches for detecting and serotyping enterovirus infections. References
Table 1 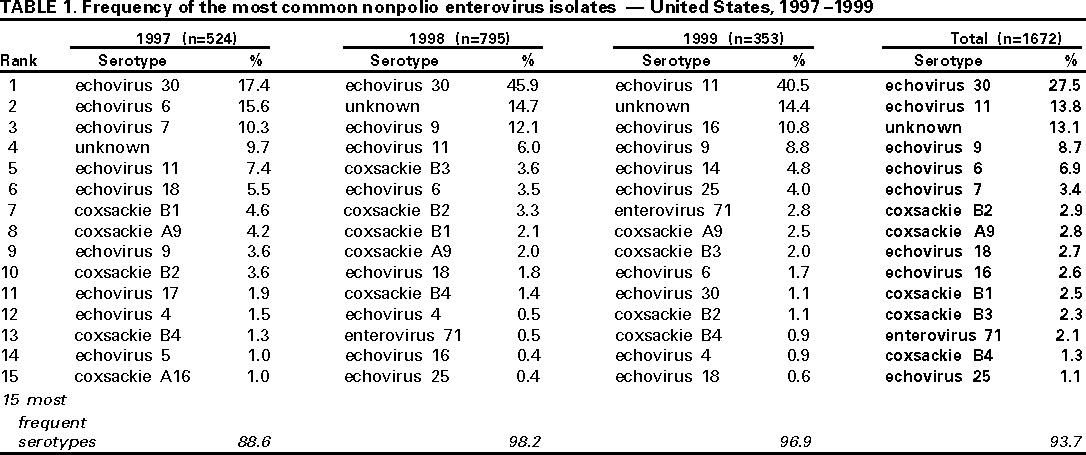 Return to top. Disclaimer All MMWR HTML versions of articles are electronic conversions from ASCII text into HTML. This conversion may have resulted in character translation or format errors in the HTML version. Users should not rely on this HTML document, but are referred to the electronic PDF version and/or the original MMWR paper copy for the official text, figures, and tables. An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S. Government Printing Office (GPO), Washington, DC 20402-9371; telephone: (202) 512-1800. Contact GPO for current prices. **Questions or messages regarding errors in formatting should be addressed to mmwrq@cdc.gov.Page converted: 10/12/2000 |
|||||||||
This page last reviewed 5/2/01
|