 |
|
|
|
|
|
|
| ||||||||||
|
|
|
|
|
|
|
||||
| ||||||||||
|
|
|
|
|
Persons using assistive technology might not be able to fully access information in this file. For assistance, please send e-mail to: mmwrq@cdc.gov. Type 508 Accommodation and the title of the report in the subject line of e-mail. Nutritional Assessment of Adolescent Refugees --- Nepal, 1999During 1990--1993, 83,000 ethnic Nepalese fled from Bhutan to refugee camps in southeast Nepal after new citizenship policies were enacted by the Bhutanese government. Although annual nutrition surveys of children aged <5 years had been conducted by international agencies, no anthropometric assessment of adolescents had been performed since the refugees arrived in 1990. After withdrawal of a fortified cereal from their rations, the number of reported cases of angular stomatitis (AS) (i.e., thinning and/or fissuring at the angles of the mouth, a sign of possible vitamin deficiency) increased six-fold during December 1998--March 1999 (from 5.5 to 35.6 cases per 1000 refugees) (Santa Tamang, MD, Save the Children Fund, United Kingdom, personal communication, 1999). The highest rates of AS were found among children and adolescents. In October 1999, CDC was invited by the World Food Programme and the United Nations High Commissioner for Refugees to assess the health status of adolescent refugees. This report summarizes the investigation, which indicated a high prevalence of low body mass index (BMI), anemia, low vitamin A status, and signs of micronutrient deficiencies among adolescent refugees. The nutritional status of a sample of refugees aged 10--19 years was assessed using anthropometry, hemoglobin measurement, laboratory testing, and a limited physical examination. Height was measured to 0.04 inches (1 mm) using 6.9 foot (2 m) height boards, and weight was measured to the nearest 3.5 ounces (100 g) using digital bathroom scales. BMI was defined as weight (in kilograms)/(height2 [in meters]). Low BMI was determined by comparing a person's BMI to that of persons of similar age and same sex in the World Health Organization (WHO) adolescent reference population (1). A fingerstick blood sample was collected to determine hemoglobin concentration using a hemoglobinometer. Anemia was defined according to WHO criteria (2). From half the participants, serum was collected by venipuncture for retinol (vitamin A) testing by high performance liquid chromatography (3,4). A retinol level <20 µg/dL was considered low (3). Participants aged 10--19 years were chosen by systematic random sampling from camp registration data of 26,235 adolescents; 400 were needed to generate prevalence estimates for low BMI and anemia. An additional 20% were chosen to ensure an adequate sample after accounting for persons who could not be located or who refused to participate. Data were analyzed using EpiInfo version 6.04b. Chi-square or Fisher's exact tests were used to compare data. For point estimates of prevalence rates, 95% confidence intervals (CIs) were calculated by the quadratic method. Of the 495 selected adolescents, 463 (94%) were enrolled; 236 (51%) were female, 253 (55%) were aged 15--19 years, and 167 (36%) (95% CI=32%--41%) had low BMI. Boys were twice as likely as girls to have low BMI (48% versus 24%; p<0.01), and participants aged 10--14 years were 1.7 times as likely to have low BMI as those aged 15--19 years (64% versus 37%; p<0.001). The prevalence of low BMI declined with age among both sexes; however, this decline was substantially more rapid among girls. Among the 458 participants who reported no recent iron tablet supplementation, 111 (24%) (95% CI=20%--28%) were anemic. The mean and median hemoglobin values were both 13.0 g/dL (standard deviation [SD]=1.7). The prevalence of anemia among girls who received no iron supplementation increased sharply after age 11 years; 33% of girls aged >12 had anemia, peaking at age 16--17 years (Figure 1) (trend test, p=0.05); 49 (37%) of 134 girls who reported having experienced menarche had anemia compared with 17 (17%) of 99 who had not (p=0.001). The prevalence of anemia among boys peaked at age 14--15 years and then decreased with age (Figure 1). Among the 190 participants assessed, 49 (26%) (95% CI=23%--30%) had retinol levels that suggested low vitamin A status. The mean retinol level was 24 µg/dL (SD=6.8) and the median was 23 µg/dL. Two participants (1%) had serum retinol values <10 µg/dL. Low serum retinol status was unrelated to age or sex. On physical examination, participants showed signs of possible micronutrient deficiencies; 133 (29%) of 463 participants had AS. One participant had spontaneous gum bleeding and 27 (6%) had gums that bled upon touch. Tingling or burning in the hands or feet during the 30 days preceding the investigation was reported by 41% of the partici pants. Although severe goiter from iodine deficiency was not found on physical examination, three participants had a grade I goiter and one had a grade II goiter (5). Reported by: World Food Programme, United Nations High Commissioner for Refugees, Save the Children Fund, United Kingdom. Div of Nutrition and Physical Activity, National Center for Chronic Disease Prevention and Health Promotion; Div of Emergency Environmental Health Svcs, and Div of Laboratory Sciences, National Center for Environmental Health; and an EIS Officer, CDC. Editorial Note:In 1992, a surveillance system was established in refugee camps in Nepal (6). This system allowed health-care providers to identify and report the increase in AS cases that led to this investigation. Although low BMI was common among refugee adolescents, the prevalence of low BMI did not exceed that of adolescents from the general population of Nepal (7). Iron deficiency may affect up to two-thirds of pregnant women in developing countries and those who enter pregnancy with adequate iron stores are more likely to complete pregnancy without developing iron deficiency (8). The findings in this report indicate that adolescent female refugees in Nepal are at risk for iron deficiency. The prevalence of low vitamin A status among participating adolescents was high. Persons aged 12--17 years may exhibit night vision problems when serum retinol levels are <20 µg/dL (9). Few participants had detectable goiter probably because iodine deficiency was avoided as a result of the distribution of iodized salt. The findings in this report are subject to at least three limitations. First, because the WHO reference population for evaluating BMI is based on data from the National Health and Nutrition Examination Survey of U.S. adolescents, the prevalence of low BMI among adolescent refugees in Nepal may be overestimated (10). Second, clinical evaluation for some micronutrient deficiencies has not been standardized. Third, the sensitivity and specificity of signs or symptoms of specific micronutrient deficiencies among adolescents has not been established. On the basis of findings from this investigation, recommended nutritional improvements for adolescents in Nepal included distributing iron and folate supplements to girls, ensuring adequate vegetable oil fortification with vitamin A, continuing surveillance for signs of micronutrient deficiencies, and adding a fortified source of micronutrients to the food ration to increase daily nutrient consumption to international standards. Long-term recommendations included the support and expansion of vegetable production in camp gardens and the raising of poultry. Since this investigation, continued surveillance for signs of micronutrient deficiencies and expansion of vegetable production in camp gardens has occurred; the addition of whole lentils to the ration is planned. References
Figure 1 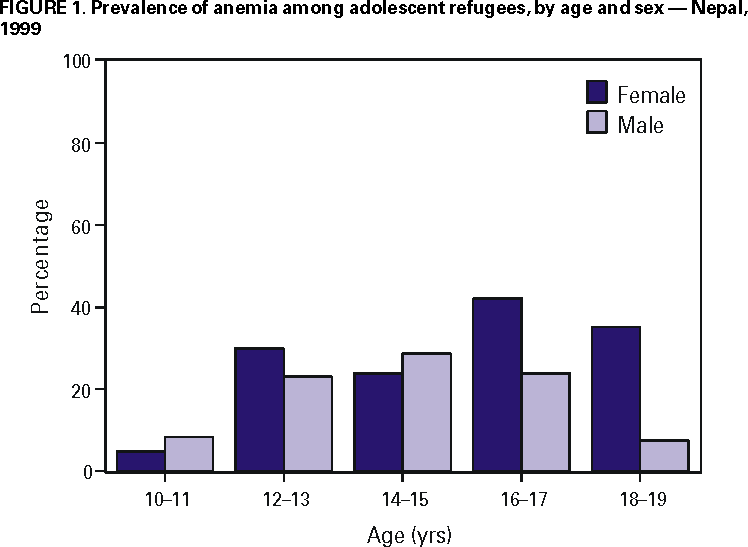 Return to top. Disclaimer All MMWR HTML versions of articles are electronic conversions from ASCII text into HTML. This conversion may have resulted in character translation or format errors in the HTML version. Users should not rely on this HTML document, but are referred to the electronic PDF version and/or the original MMWR paper copy for the official text, figures, and tables. An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S. Government Printing Office (GPO), Washington, DC 20402-9371; telephone: (202) 512-1800. Contact GPO for current prices. **Questions or messages regarding errors in formatting should be addressed to mmwrq@cdc.gov.Page converted: 9/28/2000 |
|||||||||
This page last reviewed 5/2/01
|