 |
|
|
|
|
|
|
| ||||||||||
|
|
|
|
|
|
|
||||
| ||||||||||
|
|
|
|
|
Persons using assistive technology might not be able to fully access information in this file. For assistance, please send e-mail to: mmwrq@cdc.gov. Type 508 Accommodation and the title of the report in the subject line of e-mail. Progress in Development of Immunization Registries -- United States, 1999Community-based and state-based immunization registries are confidential, population-based, computerized information systems that contain data about children's vaccinations (1) and represent an important tool to increase and sustain high vaccination coverage. Immunization registries consolidate vaccination records for children from multiple providers, provide a vaccination needs assessment for each child, generate reminder and recall vaccination notices, produce an official vaccination record, and provide practice-specific and community-based vaccination coverage assessments. One of the Healthy People 2010 national objectives is to increase to 95% the proportion of children aged <6 years who are enrolled in a fully operational population-based immunization registry (2). To assess the status of immunization registry development, CDC analyzed data from the 1999 Immunization Registry Annual Report (IRAR) of 64 jurisdictions (grantees) that receive federal immunization funds under section 317d of the Public Health Service Act. Findings from this analysis indicate that substantial progress has been made in the United States in developing and implementing community-based and state-based immunization registries. The IRAR was a self-administered questionnaire, sent to immunization program managers, that measured the degree of enrollment of a registry's target population (i.e., percentage of children in the catchment area with vaccinations recorded in the registry and percentage of public and private providers submitting records to the registry) and the implementation of 12 functional standards considered essential for immunization registry operation. The 12 standards (Table 1) were identified through a survey of immunization program managers and registry developers. Focus group research with the managers and developers was conducted to ensure consensus about the importance of these standards. Key elements associated with each standard then were identified and used to establish more sensitive registry development and implementation progress measures. In addition, the IRAR collected information on immunization registry links with other information systems. In 1999, the 64 jurisdictions (50 states, the District of Columbia, Chicago, Houston, New York City, Philadelphia, San Antonio, American Samoa, Guam, Marshall Islands, Micronesia, Northern Mariana Islands, Palau, Puerto Rico, and the U.S. Virgin Islands) were mailed the questionnaire; 62 (97%) responded. Of the 62, three (5%) grantees (all commonwealths or territories) reported no registry activity, 16 (26%) grantees reported planning or pilot-testing of registries, and 43 (69%) grantees reported implementing registries (Figure 1). Data from 37 of the 43 grantees implementing registries indicated that approximately 32% (mean=50%; median=54%) of estimated target children aged 0-5 years in the grantees' catchment areas had at least two doses of vaccine recommended by the Advisory Committee on Immunization Practices and that the information was recorded in a registry's database. Data from 42 grantees indicated that 46% (median=96%) of public providers and 13% (median=15%) of private providers had submitted records to a registry. Of the 43 grantees, all had implemented at least one key element on four of the 12 registry functional standards (i.e., electronic data storage of core data elements, protection of confidential medical information, recovery of lost data, and consolidation of vaccination records from multiple providers). Three (7%) grantees reported implementing at least one key element in each standard. However, none had implemented all key elements of the 12 functional standards (Table 1). Forty-one (95%) of the 43 grantees reported immunization registry links with at least one other health-care program; of these, 25 (61%) were linked to their state's vital records department. Links to birth certificates indicate that these registries are population-based (not provider-based or practice-based). The median number of weeks from birth to establishing a registry record was 5 weeks (range: 1-12 weeks). Reported by: Systems Development Br, Data Management Div, National Immunization Program, CDC. Editorial Note:The 1999 IRAR represents the first attempt to quantify and evaluate state-based and community-based immunization registry development in the United States. These data suggest that substantial progress has been made in U.S. communities and states in enrolling children, recruiting providers, and implementing registry functional standards. Substantial challenges remain in developing registries. One of the greatest challenges is balancing the need to protect the privacy of patients, providers, and other users of these systems with the need to gather and share information to protect the public health and provide clinical benefit to persons. In response to recommendations of the National Vaccine Advisory Committee (NVAC) 1999 report, Development of Community- and State-Based Immunization Registries (1), CDC developed specifications for privacy protection of registry participants and for the confidentiality of information contained in a registry. These specifications were approved by NVAC in February 2000. They are consistent with privacy regulations required by the Health Insurance Portability and Accountability Act of 1996 (3). Ensuring high levels of public and private provider participation in registries is a critical prerequisite to complete and accurate electronic vaccination records. In an increasingly mobile environment, where approximately 20% of children move by age 2 years (4), appropriate vaccination decision-making often depends on aggregating vaccination histories from multiple providers. Solving technical and operational challenges of sharing vaccination information between registries that may use different computer hardware and software is critical. The findings in this report are subject to at least two limitations. First, because the IRAR relies on self-reported data, some bias is expected. On-site verification of these data is planned to ensure a more accurate assessment of registry development. Second, because only immunization program grantees were surveyed, these data underestimate the degree of registry activity occurring in the United States. Survey respondents reported 84 additional immunization registries implemented at the local level. However, data collected on these systems suggest that many are not population-based. Since 1994, more than $178 million in federal funds have been awarded to state and local health departments to support the development and implementation of immunization registries (5). Fiscal savings associated with registries include avoiding duplicative vaccinations, assuring maximal returns for appointments through the use of reminder/recall notices, reducing vaccine wastage, avoiding manual generation of vaccination certificates, and avoiding manual review of multiple records to establish the Health Plan Employer Data and Information Set (HEDIS) indices. Immunization registries also can play an important role in increasing vaccine safety and monitoring adverse events because core registry data elements include vaccine date and type, manufacturer, and lot number. Registry data in Arkansas and California have been used to identify and revaccinate children who received vaccinations from sub-potent vaccine lots or an inadequate dosage of vaccine (6,7), and Oklahoma's registry data have been used to monitor the implementation of new vaccine recommendations (8). In addition, immunization registry links to broader child health information systems may help coordinate preventive care by enabling provider assessments of other health needs. Funding sources need to be identified to ensure reaching the Healthy People 2010 immunization registry objective (2). Additional information on immunization registries is available from CDC's immunization registry World-Wide Web site, http://www.cdc.gov/nip/registry; telephone (800) 799-7062; or e-mail, siisclear@cdc.gov. References
Table 1 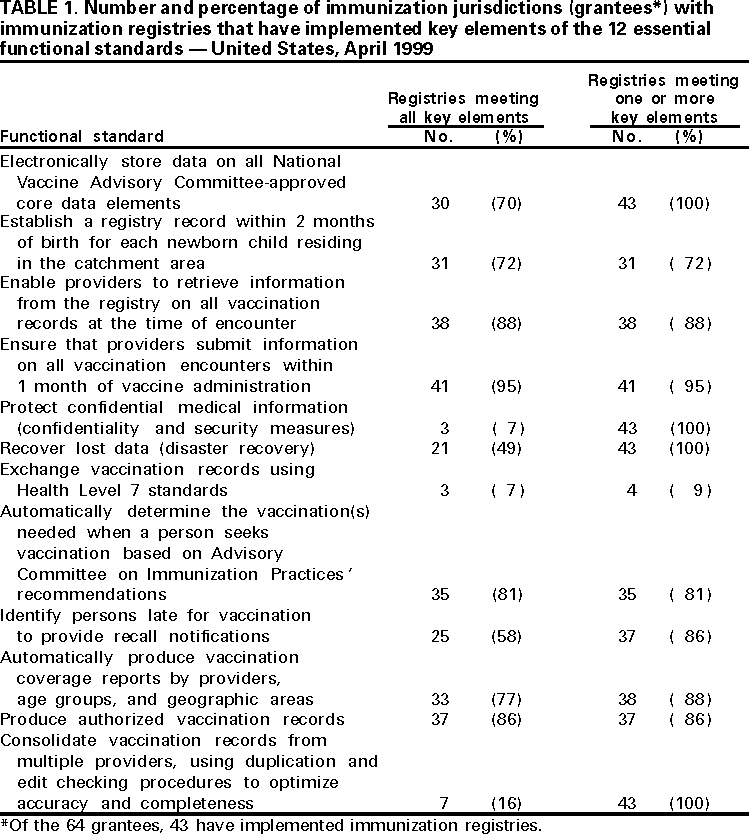 Return to top. Figure 1 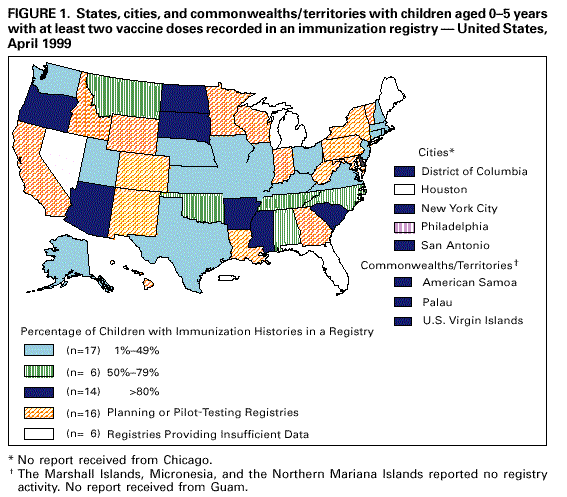 Return to top. Disclaimer All MMWR HTML versions of articles are electronic conversions from ASCII text into HTML. This conversion may have resulted in character translation or format errors in the HTML version. Users should not rely on this HTML document, but are referred to the electronic PDF version and/or the original MMWR paper copy for the official text, figures, and tables. An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S. Government Printing Office (GPO), Washington, DC 20402-9371; telephone: (202) 512-1800. Contact GPO for current prices. **Questions or messages regarding errors in formatting should be addressed to mmwrq@cdc.gov.Page converted: 4/6/2000 |
|||||||||
This page last reviewed 5/2/01
|