 |
|
|
|
|
|
|
| ||||||||||
|
|
|
|
|
|
|
||||
| ||||||||||
|
|
|
|
|
Persons using assistive technology might not be able to fully access information in this file. For assistance, please send e-mail to: mmwrq@cdc.gov. Type 508 Accommodation and the title of the report in the subject line of e-mail. Adoption of Perinatal Group B Streptococcal Disease Prevention Recommendations by Prenatal-Care Providers --- Connecticut and Minnesota, 1998Group B streptococcal (GBS) infections are the leading bacterial cause of serious neonatal disease in the United States (1). In 1996, in collaboration with the American Academy of Pediatrics and the American College of Obstetricians and Gynecologists, CDC issued consensus guidelines for preventing perinatal GBS disease (2--4). These guidelines recommend using either a screening-based or a risk-based strategy to identify women who should receive intrapartum antimicrobial prophylaxis. To assess adoption of the GBS disease prevention guidelines, the Connecticut and Minnesota state health departments surveyed prenatal-care providers during January--April 1998. This report presents the survey findings, which indicate that most prenatal-care providers in Connecticut and Minnesota have adopted perinatal GBS disease prevention policies and that strategy choice may vary by state and provider type. In Connecticut, surveys were mailed to all (n=576) licensed obstetricians/gynecologists (OBs). Group practices were allowed to submit a single response for all members. A second mailing was sent to nonrespondents. A sample of nonrespondents was then contacted by telephone to determine reasons for nonresponse. After eliminating providers from the sample who did not deliver prenatal care and those who were represented by a response from another provider in their practice, the final response rate was 77% (250 of 323). In Minnesota, surveys were mailed to a random sample of approximately 50% of practicing OBs, a random sample of approximately 25% of family physicians (FPs) who indicated on their licensure application they provided prenatal care, and all certified nurse midwives (CNMs). After three mailings, 431 (77%) of those sampled responded. The response rate was similar for all three provider groups. In 1998, most prenatal-care providers in Connecticut and Minnesota reported that their practices had a perinatal GBS disease prevention policy, although most practices did not have a written policy (Table 1). Practices in Connecticut were more likely than those in Minnesota (p<0.001) to have a GBS disease prevention policy, primarily because of the relatively low percentage of Minnesota family practices with a policy. More than 90% of individual providers from both states reported having a GBS disease prevention policy. Most providers in Connecticut chose a screening-based strategy (72%), and most in Minnesota chose a risk-based strategy (55%). When the analysis was limited to OBs in both states, OBs in Connecticut were more likely than OBs in Minnesota to choose a screening-based strategy (p<0.001). Of providers who used a screening-based strategy, 71% from Connecticut and 76% from Minnesota collected specimens from both the vagina and rectum, as recommended by the consensus guidelines. Providers using the screening-based strategy from Connecticut (82%) and Minnesota (80%) obtained cultures within 1 week of the recommended 35--37 weeks' gestation. Of providers who used a risk-based strategy in Minnesota, 80% indicated that they would administer intrapartum prophylaxis for all five of the high-risk criteria (i.e., previous infant with invasive GBS disease, GBS bacteriuria during the current pregnancy, delivery at <37 weeks' gestation, duration of rupture of membranes >18 hours, and intrapartum fever >100.4 F [>38 C]) as specified in the consensus guidelines. Questions about indications for prophylaxis under the risk-based strategy were not asked in the Connecticut survey. In Minnesota, differences were observed between the responses of FPs compared with OBs or CNMs (Table 2). OBs and CNMs were more likely than FPs (p<0.001) to report that their practices had a GBS disease prevention policy. Individual FPs were less likely to choose a risk-based strategy or to use penicillin for intrapartum prophylaxis (p<0.001 for all comparisons except strategy choice between FPs and OBs). OBs were significantly more likely than either CNMs (91% vs. 46%, p=0.001) or FPs (91% vs. 73%, p=0.03) to report collecting specimens from both the vagina and rectum. FPs were less likely to respond that they would follow all five recommended indications than either OBs (69% vs. 89%, p=0.004) or CNMs (69% vs. 84%, p=0.04). Reported by: R Lynfield, MD, K White, MPH, R Danila, PhD, Acting State Epidemiologist, Minnesota Dept of Health. A Roome, PhD, H Linardos, J Hadler, MD, State Epidemiologist, Connecticut Dept of Public Health. Respiratory Diseases Br, Div of Bacterial and Mycotic Diseases and Emerging Infections Program Network, National Center for Infectious Diseases; and an EIS Officer, CDC. Editorial Note:Perinatal GBS disease is largely preventable through targeted use of intrapartum antibiotic prophylaxis (2). Since the release of the 1996 consensus prevention guidelines, the incidence of perinatal GBS disease has declined in the United States (5). Prenatal-care providers play a critical role in preventing GBS disease. The findings in this report suggest that most prenatal-care providers in Connecticut and Minnesota have adopted one of the two GBS disease prevention strategies recom-mended in the consensus guidelines and that strategy choice may vary by state and provider type. Pregnant women should discuss GBS disease prevention with their prenatal-care providers to optimize GBS disease prevention opportunities. In Minnesota, FPs providing prenatal care were less likely than OBs or CNMs to report that their practices have a GBS disease prevention policy and to report following all the guidelines within either the risk-based or screening-based strategy. These findings suggest that additional efforts are needed to inform FPs in Minnesota about GBS disease prevention recommendations. FPs also were less likely to use penicillin, the recommended intrapartum antibiotic. Although ampicillin is an acceptable alternative (2), penicillin is preferred because it has a narrower spectrum of activity and is therefore less likely to promote antimicrobial resistance. This study was conducted before the recent shortage of penicillin G for intravenous administration. A new supplier has been identified, and penicillin G should be more available for intrapartum prophylaxis (6). In 1997, hospital obstetric departments were surveyed in both Connecticut and Minnesota about perinatal GBS disease prevention policies (7). In both states, the percentage of OBs providing prenatal care who reported adopting a perinatal GBS disease prevention policy was higher than the percentage of hospitals with a policy. Hospitals may leave decisions about GBS disease prevention activities to prenatal-care providers. Efforts to expand perinatal GBS disease prevention activities should be directed at both hospitals and prenatal-care providers (8). Although the surveys presented in this report were not designed to measure provider practices, the results suggest that prenatal-care providers are aware of the recommendations outlined in the consensus guidelines. The screening-based strategy relies on appropriate and accurate specimen collection by prenatal-care providers. Most providers in Connecticut and in Minnesota using the screening-based strategy reported collecting specimens from both the vagina and rectum. Collection site is important because vaginal/rectal specimens improve group B Streptococcus isolation rates by 40% over vaginal specimens alone (9,10). At least 80% of prenatal-care providers using the screening-based strategy in both states also reported collecting specimens at appropriate times. The risk-based strategy depends on prenatal-care providers identifying and administering prophylaxis to women at increased risk for delivering an affected infant. In Minnesota, 80% of prenatal-care providers using the risk-based strategy reported following the recommended indications for intrapartum antibiotic prophylaxis. The findings in this report are subject to at least two limitations. First, because the surveys were conducted in only two states, the results might not be generalizable to other states. Second, the surveys measured only the reported practices of prenatal-care providers and not the services actually rendered. GBS disease prevention guidelines and order forms for other information for prenatal-care providers and patients are available on the World-Wide Web at http://www.cdc.gov/ncidod/dbmd/gbs or from CDC's National Center for Infectious Diseases, Division of Bacterial and Mycotic Diseases, Respiratory Diseases Branch, Mailstop C-23, 1600 Clifton Road, N.E., Atlanta, GA 30333. References
Table 1 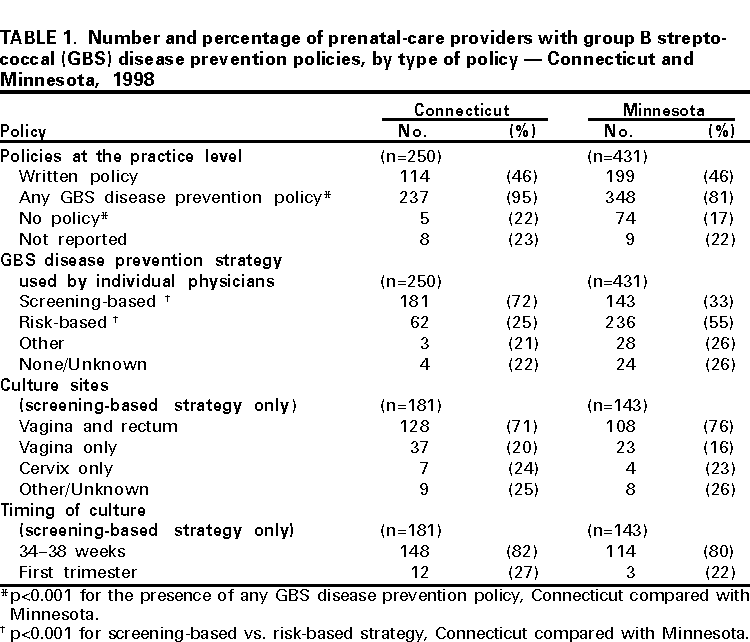 Return to top. Table 2 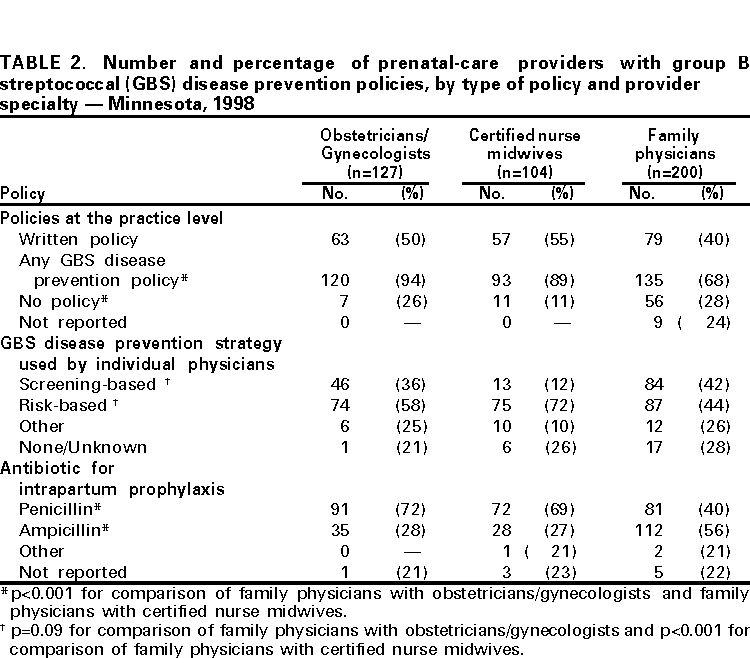 Return to top. Disclaimer All MMWR HTML versions of articles are electronic conversions from ASCII text into HTML. This conversion may have resulted in character translation or format errors in the HTML version. Users should not rely on this HTML document, but are referred to the electronic PDF version and/or the original MMWR paper copy for the official text, figures, and tables. An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S. Government Printing Office (GPO), Washington, DC 20402-9371; telephone: (202) 512-1800. Contact GPO for current prices. **Questions or messages regarding errors in formatting should be addressed to mmwrq@cdc.gov.Page converted: 3/23/2000 |
|||||||||
This page last reviewed 5/2/01
|