 |
|
|
|
|
|
|
| ||||||||||
|
|
|
|
|
|
|
||||
| ||||||||||
|
|
|
|
|
Persons using assistive technology might not be able to fully access information in this file. For assistance, please send e-mail to: mmwrq@cdc.gov. Type 508 Accommodation and the title of the report in the subject line of e-mail. Outbreak of West Nile-Like Viral Encephalitis -- New York, 1999An outbreak of arboviral encephalitis was first recognized in New York City in late August and has since been identified in neighboring counties in New York state. Although initially attributed to St. Louis encephalitis (SLE) virus based on positive serologic findings in cerebrospinal fluid (CSF) and serum samples using a virus-specific IgM-capture enzyme-linked immunosorbent assay (ELISA), the cause of the outbreak has been confirmed as a West Nile-like virus based on the identification of virus in human, avian, and mosquito samples. On August 23, 1999, an infectious disease physician from a hospital in northern Queens contacted the New York City Department of Health (NYCDOH) to report two patients with encephalitis. On investigation, NYCDOH initially identified a cluster of six patients with encephalitis, five of whom had profound muscle weakness (with axonal neuropathy by electromyelogram and requiring respiratory support [n=four]). Testing of these initial cases by IgM-capture ELISA for antibodies to the common North American arboviruses was positive for SLE virus on September 3 at CDC. Eight of the earliest case-patients were residents of a 2-by-2-mile area in northern Queens. On the basis of these findings, aerial and ground applications of mosquito adulticides and larvacides were instituted in northern Queens and South Bronx on September 3. To define the geographic extent of the outbreak, NYCDOH initiated active surveillance on August 30, and the Westchester County Department of Health and the Nassau County Department of Health initiated active surveillance on September 3. Surveillance is also ongoing in surrounding areas. A clinical case is defined as a presumptive diagnosis of viral encephalitis with or without muscle weakness or acute flaccid paralysis, Guillain-Barre syndrome, aseptic meningitis, or presence of the clinical syndrome characterizing the initial cluster of cases in a patient presenting after August 1. Before and concurrent with this outbreak, local health officials observed increased fatalities among New York City birds, especially crows. During September 7-9, officials of the Bronx Zoo noted the deaths of a cormorant, two captive-bred Chilean flamingoes, and an Asian pheasant. Necropsies performed on these birds at the zoo revealed varying degrees of meningo-encephalitis and severe myocarditis. Tissue specimens from these birds and a crow with pathologic evidence of encephalitis from New York state were sent to the U.S. Department of Agriculture National Veterinary Services Laboratories (NVSL) in Ames, Iowa, on September 10 to be tested for common avian pathogens and the equine encephalitis viruses; all tests were negative. NVSL isolated viruses from the birds' tissues and forwarded them to CDC on September 20 for identification and characterization. Testing at CDC on September 23 by polymerase chain reaction (PCR) and DNA sequencing of these isolates indicated that they were closely related to West Nile virus (WNV), which has never been isolated in the western hemisphere. In other tests at CDC, flavivirus antigen was detected in one of the autopsy specimens by immunohistochemistry, and a West Nile-like virus genomic sequence identical to that derived from the bird isolates was observed in a human brain specimen from an encephalitis case. Concurrently, specimens of brain tissue from three human encephalitis cases, forwarded by the New York State Department of Health to the University of California, Irvine, were reported as positive for West Nile-like virus sequence by genomic analysis. All serum/CSF specimens reactive to SLE by IgM ELISA were positive by WNV ELISA with higher positive/negative ratios than to SLE, and an additional 10 borderline and eight negative samples were positive for antibody to WNV. As of September 28, a total of 17 confirmed and 20 probable human cases (1) and four deaths have been reported from New York City (25 cases) and the surrounding counties of Westchester (eight) and Nassau (four). The four deaths occurred among persons aged greater than or equal to 68 years. One case-patient with onset in late August reported a history of travel to Africa completed in June 1999; none of the remaining case-patients had traveled during the incubation period to areas where WNV is known to be endemic. Two of the Westchester County case-patients had no reported travel history to New York City or other areas in which WNV previously had been detected. Onset dates ranged from August 5 to September 16 (Figure 1), although no cases had onset in New York City after control measures were extended to the entire city on September 11. The median age of case-patients was 71 years (range: 15-87 years), with the most severe clinical cases and all fatalities occurring among older persons. Vector control measures initiated in northern Queens and South Bronx on September 3 were followed by a city-wide pesticide application after laboratory confirmation of encephalitis in a Brooklyn resident with no travel history to Queens and confirmation of an additional two cases in South Bronx. According to the latest ongoing population estimates from a city-wide mosquito surveillance program, the host-seeking adult Culex pipiens mosquito population has been reduced substantially by the control operation. Following the confirmation of human cases in Westchester and Nassau counties and detection of virus in adult Culex pipiens and Aedes vexans mosquitoes and in a deceased bird from a nearby area in Connecticut, insecticide application has been initiated in these areas to reduce the mosquito population. Surveillance of wild birds and/or sentinel chickens was instituted to assess WNV distribution in the region. Emergency telephone hotlines were established in New York City on September 3 and in Westchester County on September 21 to address public inquiries about the encephalitis outbreak and pesticide application. As of September 28, approximately 130,000 calls have been received by the New York City hotline and 12,000 by the WCDH hotline. Approximately 300,000 cans of DEET-based mosquito repellant were distributed citywide through local firehouses, and 750,000 public health leaflets were distributed with information about personal protection against mosquito bites. Recurring public messages were announced on radio, television, on the New York City and WCDH World-Wide Web sites, and in newspapers, urging personal protection against mosquito bites, including limiting outdoor activity during peak hours of mosquito activity, wearing long-sleeved shirts and long pants, using DEET-based insect repellents, and eliminating any potential mosquito breeding niches. Spraying schedules also were publicized with recommendations for persons to remain indoors while spraying occurred to reduce pesticide exposure. Mosquito surveillance will continue until the first frost in New York City; Westchester, Nassau, Rockland, and Suffolk counties; and Connecticut. Surveillance for new human WNV cases will be conducted until several weeks after the first frost, when mosquito activity is expected to subside. Reported by: D Asnis, MD, R Conetta, MD, G Waldman, MD, A Teixeira, MD, Flushing Hospital, Queens; New York City acute care hospitals and microbiology laboratories; T McNamara, DVM, Wildlife Conservation Society; A Fine, MD, M Layton, MD, J Miller, MD, D Cimini, MPH, M Camilo Vargas, DVM, A Inglesby, MD, A Labowitz, K Bornschlegel, MPH, B Maldin, E Samoff, MPH, D Haddow, and the New York City Outbreak Investigation Team; S Mullin, MSW, J Gadd, MPP, E Giebelhaus, MPP, L Masuch, MSW, A Sher, M Foggin, BJ Mojica, N Cohen, MD, I Weisfuse, MD, R Bhalla, MD, E Lee, MD, D Malebranche, MD, G Sacajiu, MD, A Sharma, MD, A Ramon, MD, I Poshni, PhD, H Stirling, MPH, A Goldberg, New York City Dept of Health; J Hauer, MHS, Mayor's Office of Emergency Management, New York City; A Huang, MD, A Rosenberg, MD, P Yang-Lewis, MPH, HN Adel, MD, Westchester County Health Dept; K Gaffney, MD, A Greenberg, MD, B Smith, M Sherman, Div of Environmental Health and Disease Control, Nassau County Dept of Health; W Stone, Dept of Environmental Conservation; A Novello, MD, D White, PhD, D Morse, MD, K Spitalny, MD, R Gallo, H Leib, S Wong, MD, L Grady, MD, P Smith, MD, State Epidemiologist, New York State Dept of Health. M Cartter, MD, J Hadler, MD, Connecticut State Health Dept. WI Lipkin, MD, T Briese, PhD, XY Jia, MD, Emerging Diseases Laboratory, Univ of California, Irvine. National Veterinary Svcs Laboratories, Animal and Plant Health Inspection Svc, US Dept of Agriculture, Ames, Iowa. State Br, Div of Applied Public Health Training, Epidemiology Program Office; Meningitis and Special Pathogens Br, Div of Viral and Rickettsial Diseases, and Arbovirus Diseases Br, Div of Vector Borne Infectious Diseases, National Center for Infectious Diseases; and EIS officers, CDC. Editorial Note:WNV is a flavivirus belonging taxonomically to the Japanese encephalitis subgroup that includes the serologically closely related SLE virus, Kunjin virus, Murray Valley encephalitis virus, and others. WNV was first isolated in the West Nile Province of Uganda in 1937 (2). The first recorded epidemics occurred in Israel during 1950-1954 and in 1957. Epidemics have been reported in Europe in the Rhone delta of France in 1962 and in Romania in 1996 (3-5). The largest recorded epidemic occurred in South Africa in 1974 (6). It is unclear whether the virus that caused this outbreak is a previously identified strain of WNV or a new variant. The genomic sequences identified to date from a human brain, virus isolates from zoo birds, and viruses isolated from a dead crow and two mosquito pools from Connecticut appear identical. Based on preliminary serologic testing, this outbreak was originally believed to be caused by the SLE virus. SLE and West Nile viruses are antigenically related, and cross reactions are observed with some serologic tests. Results of PCR-based sequencing that identified WNV prompted more specific testing. The IgM-capture ELISA used in testing serum/CSF samples in this outbreak is rapid, sensitive, and quantitative. The limitations of some serologic assays emphasize the importance of isolating the flavivirus from entomologic, clinical, or veterinary material. The availability of virus isolates and genomic sequences from birds and human brain tissue permitted the discovery of this West Nile-like virus in North America. Although it is not known when and how a West Nile-like virus was introduced into North America, international travel of infected persons to New York or transport by imported infected birds may have played a role. WNV can infect a wide range of vertebrates, but in humans it usually produces either asymptomatic infection or mild febrile disease. Within its normal geographic distribution of Africa, the Middle East, western Asia, and Europe, WNV has not been documented to cause epizootics in birds; crows with antibodies to WNV are common, suggesting that asymptomatic or mild infection usually occurs among crows in those regions. Similarly, substantial bird virulence of SLE virus has not been reported. Therefore, an epizootic producing high mortality in crows and other bird species is unusual for either WNV or SLE virus and may represent introduction to a native bird population or a new virulent strain. For both viruses, migratory birds may play an important role in the natural transmission cycles. Like SLE virus, WNV is transmitted principally by Culex species mosquitoes, but also can be transmitted by Aedes, Anopheles, and other species. The predominance of urban Culex mosquitoes trapped during this outbreak suggests an important role for this species. Enhanced monitoring through surveillance for early detection of this virus outside of the affected area will be crucial to guide extension of control measures. References
Figure 1 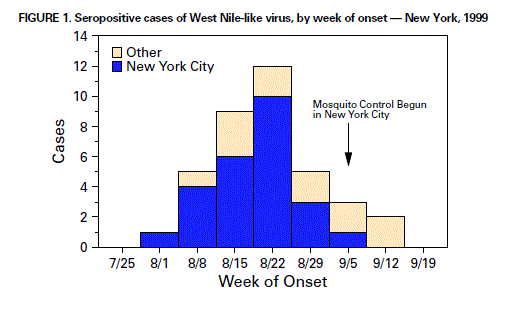 Return to top. Disclaimer All MMWR HTML versions of articles are electronic conversions from ASCII text into HTML. This conversion may have resulted in character translation or format errors in the HTML version. Users should not rely on this HTML document, but are referred to the electronic PDF version and/or the original MMWR paper copy for the official text, figures, and tables. An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S. Government Printing Office (GPO), Washington, DC 20402-9371; telephone: (202) 512-1800. Contact GPO for current prices. **Questions or messages regarding errors in formatting should be addressed to mmwrq@cdc.gov.Page converted: 9/30/1999 |
|||||||||
This page last reviewed 5/2/01
|