 |
|
|
|
|
|
|
| ||||||||||
|
|
|
|
|
|
|
||||
| ||||||||||
|
|
|
|
|
Persons using assistive technology might not be able to fully access information in this file. For assistance, please send e-mail to: mmwrq@cdc.gov. Type 508 Accommodation and the title of the report in the subject line of e-mail. HIV Prevention Through Early Detection and Treatment of Other Sexually Transmitted Diseases -- United States Recommendations of the Advisory Committee for HIV and STD PreventionSummary In May 1997, the Advisory Committee for HIV and STD Prevention (ACHSP) reviewed data on the relation between curable sexually transmitted diseases (STDs) and the risk for sexual transmission of human immunodeficiency virus (HIV). ACHSP considered that the evidence was strong that early detection and treatment of other STDs is an effective strategy for preventing sexually transmitted HIV infection but was concerned that this strategy has not been clearly articulated or implemented as a core strategy for HIV prevention in the United States. In the context of persistently high prevalence of STDs in many parts of the United States and with emerging evidence that the U.S. epidemic of HIV infection and acquired immunodeficiency syndrome (AIDS) increasingly is affecting population groups with the highest rates of curable STDs, ACHSP recommends the following actions:
INTRODUCTION The Advisory Committee for HIV and STD Prevention (ACHSP) provides oversight and guidance to CDC in the prevention of human immunodeficiency virus (HIV) -- the virus that causes acquired immunodeficiency syndrome (AIDS) -- and other sexually transmitted diseases (STDs). On May 2, 1997, ACHSP reviewed data on the role of STD detection and treatment in the prevention of HIV infection. Based on this review, ACHSP concluded that early detection and treatment of curable STDs should be implemented more widely as an HIV prevention strategy in the United States. CDC is disseminating these ACHSP recommendations to HIV prevention community planning groups, prevention specialists, and policymakers who address HIV and STD prevention. ACHSP also notes that early detection and treatment of STDs should be only one component of a comprehensive HIV prevention program, which also must include a range of social, behavioral, and biomedical interventions. Furthermore, a comprehensive national program for STD prevention must address other health concerns (e.g., STD-related infertility or adverse outcomes of pregnancy), and it requires diverse activities that go beyond early STD detection and treatment. Also, these recommendations focus on the major treatable STDs -- genital chlamydial infections, gonorrhea, syphilis, and chancroid -- because of the strong evidence of their cofactor role in HIV transmission. Also, prevention programs and routine public health surveillance for these conditions already exist in the United States. However, several studies indicate that treating other STDs (e.g., genital herpes infections and trichomoniasis) and genital tract syndromes related to sex (e.g., bacterial vaginosis) also can help prevent HIV transmission. BACKGROUND Curable STDs as Cofactors for HIV Transmission Epidemiologic Evidence Since the beginning of the AIDS epidemic, researchers consistently have noted a strong epidemiologic association between HIV/AIDS and other STDs in developing and industrialized countries, including the United States (1,2). The mutually reinforcing nature of these infectious processes has been termed "epidemiological synergy" (1). Diverse observational studies, including cross-sectional studies and cohort studies of HIV seroconvertors, have indicated at least a twofold to fivefold increased risk for HIV infection among persons who have other STDs, including genital ulcer diseases and nonulcerative, inflammatory STDs (3-12). These "STD cofactor effects" were corroborated for each of the major specific genital ulcer pathogens -- Treponema pallidum (the agent of syphilis), Hemophilus ducreyi (the agent of chancroid), and herpes simplex virus type 2 (HSV-2, the agent of genital herpes) -- as well as for the pathogens principally responsible for nonulcerative STDs
Biologic Mechanisms Several studies have explored potential biologic mechanisms by which other STDs can facilitate sexual transmission of HIV infection by increasing infectiousness or susceptibility. HIV is detected routinely in the exudate of genital ulcers from HIV-infected men and women (14-17). Ulcers bleed easily and can come in contact with vaginal, cervical, oral, urethral, and rectal mucosa during sex. In men and women, inflammatory STDs (e.g., gonococcal and chlamydial infections) appear to increase both the prevalence of HIV shedding and the HIV RNA copy number or "viral load" in genital secretions (17-20). Thus, these STDS are likely indicators of HIV infectiousness (1,21). In HIV-infected men, gonococcal infection increases shedding of HIV RNA in semen tenfold, but effective treatment of gonorrhea rapidly reduces HIV shedding to background levels (20). In addition, both ulcerative (e.g., herpes, syphilis, and chancroid) and nonulcerative STDs (e.g., gonorrhea and chlamydia) attract CD4+ lymphocytes to either the ulcer surface (22) or the endocervix (23), which disrupts epithelial and mucosal barriers to infections and establishes a potential mechanism to increase a person's susceptibility to HIV infection. Intervention Trials To test these epidemiologic and biologic findings, two community-level, randomized controlled intervention trials have been conducted. One trial, in the Mwanza district of Tanzania, documented that continuous provision of improved STD treatment reduces the acquisition of HIV infection (24). In that study, providing effective drugs for STDs and training health-care providers to treat symptomatic STDs resulted in a 38% lower HIV incidence in six intervention communities compared with six matched control communities (Figure_1). This lower HIV incidence was not accompanied by changes in sexual behavior or by condom use that might confound the direct association between improved STD treatment and lowered HIV incidence. This randomized controlled trial (RCT) was the first documented intervention that successfully reduced HIV incidence at the population level. This study suggests that treatment of symptomatic STDs is an effective, community-level strategy for HIV prevention in settings and subpopulations in which HIV infection and other STDs are prevalent. Moreover, the program's cost-effectiveness of $217 (U.S.) per HIV infection averted and $10 (U.S.) per disability-adjusted life-year (DALY) saved, compared favorably with other highly effective public health interventions (e.g., childhood vaccinations, which costs $12-$17 {U.S.} per DALY) (25). The second trial took place in the Rakai District of Uganda and used an alternative approach: intermittent mass STD treatment administered in a blinded fashion every ten months (26), as opposed to the continuous, enhanced treatment of symptomatic STDs in Mwanza, Tanzania. Early results of the Rakai study indicate no difference in the incidence of HIV infection between intervention and control areas, despite significant reductions in curable STDs in the intervention areas (27). Important differences distinguish the two studies. These differences could help the public health community assess which STD interventions are most effective in influencing HIV transmission. An important factor may be continuous efforts compared with intermittent or episodic service delivery, even when the latter is highly intensive. A second could be the stage of an HIV epidemic: at the time of the first trial, Mwanza was experiencing a relatively early HIV epidemic, with community HIV prevalence of 4%, whereas the Rakai study took place in one of the world's most mature epidemics, with a community HIV prevalence of approximately 16% (28). These factors and others could have contributed to the differing outcomes of these studies, and additional investigation is needed. The results of these two intervention trials reinforce the importance of ongoing operational research to identify the strategies most effective for HIV prevention (29). Public health officials should assess the epidemiologic context of the HIV and STD epidemics based on the available data (i.e., epidemiologic associations, biologic mechanisms, and intervention trial results) to plan and monitor implementation of early detection and treatment of STDs to prevent HIV infection in the United States. Other Strategies for Reducing STDs for HIV Prevention Mathematical modeling of the biologic effects of other STDs on HIV infectiousness and susceptibility has documented that standard epidemiologic measures of effect (e.g., odds ratios or relative risks) might substantially underestimate the effect of STDs on HIV transmission (30,31). This underestimation occurs because standard measures do not consider the effects of STD cofactors on ongoing HIV transmission. Models suggest that STD incidence and prevalence could be critical determinants of whether sustained heterosexual HIV epidemics can persist in subpopulations with different levels of risky sexual behavior (32). Modeling also suggests other findings relevant to the role of STD treatment in a comprehensive approach to HIV prevention. First, models have demonstrated a substantially greater effectiveness and cost-effectiveness when STD prevention is implemented early in an HIV epidemic, before widespread dissemination of infection (33-36). The greater impact of the Tanzania study (24) compared with the Ugandan study (27) appears to corroborate this prediction. Second, directing STD interventions toward persons at highest risk for acquiring and transmitting infection with HIV and other STDs will generate a greater impact on the subsequent course of an epidemic (34, 36, 37). Empiric evidence of these findings is available from interventions that improved STD services for female sex workers in countries where HIV transmission was strongly associated with commercial sex (10,38). Intersecting Epidemics of HIV Infection and Other STDs As discussed previously, concurrent STDs increase the transmission probability for HIV infection. In addition to this STD cofactor effect, the impact of other STDs on HIV transmission will depend on a) the magnitude of the epidemics of other STDs in the population and b) the extent to which the epidemiology of curable STDs overlaps that of HIV infection. Magnitude of STD Epidemics in the United States The United States has the highest rates of STDs in the industrialized world (39). In 1996, approximately 400,000 genital C. trachomatis infections were detected and reported to CDC (40), making this infectious disease the most commonly reported in the United States (41), despite continuing evidence that screening is limited even among the highest risk groups (42,43). Although gonorrhea incidence in the United States declined nearly 60% during 1980-1996 (40,44), the 1996 rate of 124/100,000 was 26 times greater than the rate in Germany (4.7) and 50 times the rate in Sweden (2.4). The total rate of syphilis in the United States in 1996 was 20.2/100,000 -- 13 times higher than the rate in Germany (1.5) and 33 times higher than the rate in Sweden (0.6). Although it is not a curable STD, the prevalence of infection with HSV-2 (a chronic, persistent viral infection) actually increased by 30% during the first 15 years of the AIDS epidemic in the United States; by 1991, a total of 22% of all U.S. adults, an estimated 45 million persons, were infected with HSV-2 (45). This often underrecognized burden of STDs in the United States led the Institute of Medicine to issue a landmark report on STDs and their prevention in the United States (39). In this report, the Institute of Medicine estimated the 1994 cost of sexually transmitted HIV infection at $6.7 billion and the cost of other STDs and their immediate sequelae at $10 billion (39). Against this backdrop of high overall STD rates and costs, some subpopulations experience even higher-than-average incidence and prevalence of STDs. Sexually active adolescents in most parts of the United States, regardless of race or socioeconomic status, have a point prevalence for chlamydial infection of 5%-10% (46). In 1996, routine notifiable disease reporting alone indicated a gonorrhea case rate of 3% for African-American women aged 15-19 years and men aged 20-24 years in the United States (40). Reported rates of primary and secondary syphilis in the United States are approximately fiftyfold higher among African Americans than whites (40). Men who have sex with men (MSM), especially young MSM, continue to have high rates of STDs (47,48). STD prevalence rates also are typically high among persons who use illicit drugs, including both injecting-drug users (IDU) and noninjecting-drug users. In terms of geographic variation, bacterial STD rates are higher in many large cities and sharply higher in the southeastern United States than for the country as a whole (40,49). Superimposed on the high prevalence of STDs overall in the United States, the higher rates within these demographic or geographic subgroups suggest that the potential for reducing STD prevalence in these groups could be especially large. Intersecting Epidemiology of HIV Infection with Curable STDs The potential impact of STDs in facilitating HIV transmission depends not only on the magnitude of the STD cofactor effects and the overall STD prevalence rates, but also on the extent to which other STDs are concentrated disproportionately among persons and subpopulations likely to be exposed to HIV infection. STD/HIV coinfection rates can be one indicator of this epidemiologic interaction, which heightens the potential contribution of curable STDs to the sexual transmission of HIV infection. For example, a much higher prevalence of HIV coinfection exists among persons with any STDs than among those without STDs or a history of STDs (1,50-52). Consequently, interventions directed toward any person with an STD are targeted intrinsically to persons with a higher prevalence of and at higher risk for HIV infection. Among persons with STDs, the likelihood of HIV coinfection typically is high among persons with ulcerative STDs, reflecting shared risk factors and the strong, mutually reinforcing effects of ulcerative STDs and HIV infection on ulcer persistence and HIV transmissibility (1). For example, a recent multicenter study of syphilis therapy in the United States documented an 18% prevalence of HIV infection among patients with early syphilis in several large cities in the United States (53). A study from New York (city), which has a longstanding HIV/AIDS epidemic, reported a tendency toward increasing HIV prevalence over time among genital ulcer disease patients, even in an STD clinic setting with declining overall rates of HIV infection (50). A newly reemerging syphilis epidemic in Baltimore was concentrated among HIV-infected persons, with HIV/syphilis coinfection rates of 18% -- higher than the 3% HIV prevalence observed among other STD clinic patients (54). These examples reinforce the need to detect, treat, and prevent bacterial ulcerative STDs wherever they persist (55) or reemerge (56) in a community. Although HIV coinfection rates typically are higher-than-average among persons with ulcerative STDs, the high incidence and prevalence of the major nonulcerative STDs, especially chlamydia and gonorrhea (40), suggests that their population-attributable risk for promoting sexual transmission of HIV infection could be even greater (1). Moreover, data from the Supplement to HIV/AIDS Surveillance (SHAS) project and other studies demonstrate that, despite the markedly high prevalence of HIV infection among genital ulcer disease patients, the incidence of nonulcerative STDs among HIV-infected persons could be higher than the incidence of genital ulcer disease (57,58). In addition to these considerations related to persons infected with different STD pathogens, subpopulations at increased risk for HIV transmission typically have higher rates of STDs. For example, despite substantial declines since the beginning of the AIDS epidemic, MSM continue to have high rates of bacterial and other STDs (48,59), and outbreaks of gonorrhea continue to occur (47). Notably, the occurrence of other STDs continues to be an important predictor of HIV seroconversion among young MSM (59). Also, although parenteral exposure through contaminated injection equipment is paramount among IDUs, they are at risk for sexual HIV transmission, as well. In one study of female IDUs, for example, syphilis was identified as a prominent risk factor for acquiring new HIV infection, a finding that suggests sexual transmission could account for an underrecognized subset of new HIV infections in this group (60). Recent Shifts in the HIV/AIDS Epidemic in the United States The U.S. HIV/AIDS epidemic has evolved recently in three ways that suggest that STD cofactor effects are becoming increasingly important. First, heterosexual HIV transmission is responsible for the most rapidly increasing subset of U.S. AIDS cases, having increased both proportionately and absolutely (61), despite recent evidence that the epidemic is leveling off within some other subpopulations (62). Heterosexual HIV transmission is particularly important among women less than 25 years, accounting for more than half of AIDS cases in this group in 1996 (61). As noted previously, evidence exists for a prominent STD cofactor effect related to heterosexual HIV transmission (1,2,24,63,64). Second, the most striking recent subpopulation increase in AIDS in the United States is among women, particularly young African-American women (61,62), among whom the prevalence of other STDs also is disproportionately high (40). The shift in the HIV/AIDS epidemic toward African Americans reflects, and could in part be attributable to, the long-standing disproportionate burden of other STDs in this group. It also is closely related to the increasing prevalence of heterosexual HIV transmission, which reinforces the importance of routine screening for asymptomatic STDs, because the proportion of STDs that are asymptomatic is higher among women than men. Third, an increasing proportion of all AIDS cases (62) and AIDS cases among young women (61) are being reported in the southeastern United States, a trend that reflects the geographic distribution of notifiable STDs (e.g., gonorrhea and syphilis) nationwide (Figure_2) (40,44,49). Like other trends, the geographic overlap between U.S. regions (e.g., the South) that have the highest STD rates and those with the most rapidly expanding epidemic of heterosexual AIDS and HIV infection (61,64) suggests a need to strengthen early STD detection and treatment among persons at risk for HIV infection. Current Status of STD Clinical Services in the United States Access to and Quality of Care in STD Clinics Widespread availability of good-quality clinical STD services is essential to ensuring that infections are detected and treated to help reduce the risk for STD and HIV transmission (39). However, persons living in the United States currently have limited awareness of their need for STD services, as well as limited access to these services. Nearly one out of five persons living in the United States think that all STDs are curable, and more than half do not know that other STDs facilitate HIV transmission (39). Only half of local public health departments in the United States provide STD preventive services, compared with 96% that provide vaccinations (Figure_3) (65). Even where STD services are provided, access to care often is restricted by limited hours of operation and the lack of timely services (66). Nearly 40% of local health departments that provide STD services cannot see potentially infected (and infectious) new patients the same day they seek care, and 15% cannot see such patients for 3 days or more (65). STD Services Outside Public Health Clinics As a result of changing health-care systems in the United States, most patients with STDs, especially women, are not examined in public STD clinics. In primary-care settings, even if persons are examined by a clinician, most providers do not routinely obtain a sexual history or ask about or screen for STDs (42,67). To address this problem, innovative approaches to delivering STD clinical care outside of categorical STD clinics are being explored. Integration of STD care and family-planning services within a broader reproductive health model provides efficient health care for women and has been highly successful as a primary strategy for reducing chlamydial infections in the United States (68). Prenatal and obstetrical-care settings also provide a venue for STD/HIV screening and prevention, while enhancing the potential for prevention of perinatal HIV transmission (69) and other STD-related adverse outcomes of pregnancy (70,71). Recognition that public-sector STD services cannot reach all persons who need them has prompted additional efforts to reach private providers (72,73). Specific strategies include promoting improved STD services within managed care organizations (MCOs) (74), which are emerging as a dominant medical-care system in much of the United States (75), particularly for the more disadvantaged subset of the population at higher risk for HIV infection. For example, the role of MCOs was specifically considered in the development of the Institute of Medicine report, The Hidden Epidemic, and MCO representatives helped develop the 1998 Guidelines for Treatment of Sexually Transmitted Diseases, which is used to set STD practice standards in the United States (76). Additional efforts have been undertaken with providers who care for other critical subpopulations with high rates of STDs (e.g., adolescents) (73), but these efforts need to be increased substantially. Establishing STD clinical services in nonclinical, institutional, or community settings typically is more expensive than clinic-based approaches but could yield substantial benefits if services are extended to persons at higher-than-average risk for acquiring or transmitting STDs within communities (77,78). New STD diagnostic methods that use urine, self-collected swabs, or other noninvasive specimens will permit direct outreach to persons who might not effectively access any formal health-service setting (79,80). In addition, the recent approval of rapid HIV tests and tests that use oral fluids as a specimen (81-83) could facilitate the widespread use of HIV testing and counseling in STD care settings, particularly nontraditional ones. Community outreach for STD prevention is limited in many jurisdictions (Figure_3) (65), particularly outside dedicated STD clinics or in other venues where there are persons at higher-than-average risk for STD or HIV infection. For example, although U.S. prisons have expanded screening programs for HIV infection in recent years, STD screening and prevention remains less common (84). Fewer than half of U.S. jails (85) offer routine STD detection and treatment programs, despite their documented high yield and impact (77,86). Importance of Asymptomatic Infections An often unrecognized aspect of STDs, including bacterial STDs, is how frequently persons with these infections have no symptoms or do not recognize symptoms. Most studies of STDs are conducted in health-care settings specifically for persons who do recognize symptoms; therefore, these studies usually overestimate the proportion of infected persons who are symptomatic. Studies of STD screening in nonhealth-care settings (e.g., jails, workplaces, and communities) or health-care settings where STD treatment is not the primary function (e.g., family-planning clinics) suggest that most persons with gonorrhea or chlamydia are asymptomatic. Among women seeking contraceptive or other gynecologic services, 52% of those with gonorrhea and at least 70% of those with chlamydial infection exhibited neither symptoms nor signs of infection (87,88). Four population-based studies of men documented that 68%-92% of those with gonorrhea reported no symptoms (89-92), and one study reported that 92% with chlamydia reported no symptoms (92). This common lack of symptoms for gonorrhea and chlamydia has important implications for treatment of these STDs, as well as for the way in which STD treatment can be used for HIV prevention. Providing access to treatment for persons with STD symptoms is an essential aspect of STD and HIV prevention, but most curable STDs will go unrecognized and untreated without increased efforts to detect and treat persons without symptoms. Opportunities to identify and treat asymptomatically infected persons include screening in health-care settings when persons are present for other problems (e.g., in emergency rooms or family-planning clinics, during routine or annual physical examinations, and during vaccination visits for adolescents and adults) and in nonhealth-care settings (e.g., schools and jails). Screening also can be conducted through sex-partner-notification programs. RECOMMENDATIONS Initial Steps to Enhance STD Detection and Treatment Considering the data presented previously, ACHSP recommends that early detection and treatment of curable STDs that facilitate HIV transmission should be a central and explicit component of national, state, and local strategies to prevent HIV infection and AIDS. Although enhancing STD screening and treatment has always been desirable as a way to prevent the complications of STDs, current knowledge indicates it also is critical to preventing HIV infection. Any activity that decreases the incidence and prevalence of STDs in a population will decrease the prevalence of this key cofactor and should therefore decrease HIV transmission. Thus, health-care providers could prevent HIV transmission not just by treating STDs among persons with HIV infection, but also by treating and preventing STDs among any persons at risk for STDs. Other strategies to help achieve these goals are improving access to and quality of STD clinical services, expanding screening and treatment for STDs in medical settings, and establishing or expanding screening for STDs in nonmedical settings. Initial steps for improving sexually transmitted disease (STD) detection and treatment to prevent human immunodeficiency virus (HIV) transmission
Improving Access to and Quality of STD Clinical Services A basic step toward implementing this strategy is to provide timely, good-quality STD clinical care to persons who recognize or suspect symptoms of STDs or who suspect they have been exposed and seek STD clinical care on their own. A major component of the randomized controlled trial in Mwanza was the simple enhancement of the quality of clinical STD services for symptomatic persons, which was recognized by the population and translated rapidly into increased use of services (24). Better and faster methods are needed to assess effective access to STD clinical care in communities, and strategies are needed to extend services rapidly to those in need and at risk for HIV and STD transmission. Accordingly, ACHSP makes the following recommendations:
Enhanced Screening for STDs in Medical Settings Because most STDs are asymptomatic, voluntary care-seeking specifically for STD-related symptoms is unlikely to lead to detection of most infections. Thus, STD screening programs are a critical component of expanding early detection and treatment. Although many persons at risk for STDs cannot or do not access health-care services specifically for STD testing and treatment, they often do visit several health-care settings for other purposes. Such visits currently represent missed opportunities to diagnose and treat STDs and to decrease transmission of HIV infection. In 1996, the U.S. Public Health Service published national guidelines for screening for syphilis, gonorrhea, chlamydial infection, and genital herpes (93). Other national organizations also have issued guidelines for screening specific population groups, such as adolescents (95). These guidelines were developed to prevent the complications of STDs themselves and generally do not account for the individual or population-level risk for HIV infection caused by the presence of these STDs in individuals or communities. Because of the impact of HIV disease on individuals and communities, ACHSP endorses the existing screening guidelines and extends them to include the following recommendations:
Counseling Persons with HIV/AIDS and a New STD The presence of a new STD in a person with HIV/AIDS strongly suggests unprotected sex, a behavior that could place another person or persons at risk for HIV infection. Counseling should consist of several components, including the following:
In addition to these counseling messages, any newly or previously identified person with HIV/AIDS who is not in a high-quality HIV/AIDS treatment program should be referred to one. Expanded STD Screening in Nonmedical Settings Many persons at increased risk for STDs and HIV infection visit health-care providers infrequently, and some populations are easier to reach outside traditional clinical settings. Newer screening tests (i.e., those using urine samples or self-obtained swabs) make screening in nonmedical settings increasingly feasible. ACHSP makes the following recommendations for STD screening in nonmedical settings:
Presumptive Treatment for STDs Persons with positive tests for STDs often are difficult to locate when the results become available, and even when they are found, they have had the opportunity to transmit the infection during the interval between testing and treatment. Because of this risk and because of the safety of the antibiotics used to treat curable STDs, persons likely to have these STDs should be treated presumptively while awaiting laboratory confirmation. Presumptive antibiotic treatment for STDs has been part of CDC's STD treatment guidelines for many years (69). ACHSP endorses these guidelines, viewing them now as part of a national strategy for HIV prevention, especially in settings where the likelihood of STD infection is high or prompt follow-up for subsequent treatment is in question. These guidelines include the following recommendations:
Behavioral Issues Related to Early Detection and Treatment of STDs Although early STD detection and treatment essentially represents a biomedical tool for lowering the risk for sexual transmission of HIV infection, important associated behavioral issues exist. The most important new messages for persons at risk for HIV infection and other STDs include a) other STDs facilitate HIV transmission, and early STD detection and treatment is an HIV prevention strategy; b) recognizing and watching for the symptoms of STDs is important; and c) most STDs produce no symptoms, so routine screening is crucial. A complementary set of messages should be developed and disseminated to health-care providers, and specific information on where to obtain quality STD services should be available to persons who need it. These behavioral messages should supplement, not supplant, messages already emphasized in HIV-prevention counseling, such as the advantages of reducing the number of sex partners, the importance of knowing the HIV serostatus of one's partner(s), the importance of consistent and correct condom use, and the need to develop and implement strategies for avoiding risky sexual and other behaviors. ADDITIONAL SUPPORTIVE ACTIVITIES HIV Screening Among Persons with Other STDs Screening for HIV infection among persons with other STDs is an important HIV prevention strategy. Although HIV counseling and testing among persons with other STDs has long been recommended and applied in the United States (97), the extent of the practice of this preventive service varies and is limited in many jurisdictions. A person could be more receptive to HIV prevention messages delivered when an STD is diagnosed. Therefore, broader practice of HIV counseling and testing among STD patients, although not strictly pertinent to the strategy of early STD detection and treatment, could provide an opportunity to reinforce awareness of the cofactor role of STDs for HIV transmission and the importance of seeking timely medical care for STD symptoms. It also provides an important opportunity to assess coinfection rates for HIV infection and other STDs. However, these services should be implemented with scrupulous attention to the quality of the counseling and with adequate referral systems to redirect HIV-seropositive and at-risk HIV-seronegative persons into long-term primary-care, prevention, and drug treatment services, as appropriate. Community-Level Prevention of the Highest Risk STDs Some less common STDs in the United States have been associated with a higher-than-average prevalence of HIV coinfection and transmission risk. Examples include rectal gonorrhea among MSM and the bacterial genital ulcer diseases (syphilis and chancroid). Rectal gonorrhea in men should be monitored carefully, and its persistence should be considered a community-level sentinel event reflecting a mixture of higher-risk behavior, STD cofactor effects, and other HIV transmission risk factors. It should prompt an urgent HIV prevention response, including but not restricted to enhanced STD detection and treatment among MSM. Also, because of the strong impact of syphilis and chancroid on HIV transmission, U.S. public health officials are developing and implementing plans to eliminate domestic transmission of syphilis (98). This program could be particularly important because of the apparent cyclical nature of syphilis epidemics in the United States in the absence of concerted efforts toward elimination of this disease (49,55). Therefore, ACHSP supports syphilis elimination as a potentially high-impact activity leading to reduced STD-facilitated HIV transmission in the United States. It recommends that STD and HIV prevention programs collaborate in the development and implementation of syphilis elimination plans in their jurisdictions. Improving and Using STD Surveillance for HIV Prevention Early detection and treatment of curable STDs as an HIV prevention strategy also has implications for public health surveillance of STDs. Improved quality, completeness, and timeliness of STD surveillance can provide critical information to target early STD detection and treatment and help target HIV prevention strategies. If reporting requirements are met, expanded early detection of STDs within a jurisdiction should lead to more complete STD surveillance data, which could be an important element of the epidemiologic profile used by HIV prevention community planning groups (99). Although it also could lead to an increase in reported STD rates in the initial years of expanded services, this result should be seen as a positive indicator of enhanced early detection of STDs. The rates should decline in subsequent years. In addition to these general concerns, several surveillance issues are important to improving early STD detection and treatment for HIV prevention. In many areas of the United States and nationally, data are not collected systematically on the anatomic site of infection for persons with gonorrhea. However, gonorrhea among MSM, especially rectal gonorrhea, can be an important indicator of the potential for HIV transmission. Therefore, the anatomic site of gonococcal infection should be reported consistently as part of routine notifiable disease surveillance in all jurisdictions. Enhanced STD surveillance also should include monitoring the prevalence of STDs and HIV infection in settings where there are persons at high risk for both (e.g., correctional facilities and drug treatment centers) (77,86,100,101). This latter surveillance strategy complements the expanded early detection and treatment of STDs in settings where higher-risk persons are encountered. The observed prevalence of STD and HIV infections in these specific venues, as well as additional data when available on STD/HIV coinfections, should be used to guide further program interventions (100). Finally, HIV counseling and testing data systems should be modified to ensure that STD diagnoses are captured. Cross-Training HIV and STD Prevention Staff Implementing the strategy of enhanced STD detection and treatment for HIV prevention is likely to require or be enhanced by greater mutual familiarity and sense of shared purpose within state and local HIV and STD prevention programs. Cross-training program and management staff in the current practices, technology, and guidelines of the other program should mutually strengthen both HIV and STD prevention programs. In many jurisdictions and/or for certain subpopulations, cross-training could be an initial activity to help adapt these general recommendations to the specific epidemiologic, health-care, and prevention service needs of the local population. Potential Role of Other STDs Although this report has emphasized early detection and treatment of curable, especially bacterial, STDs, other STDs and related conditions also warrant appropriate management and could constitute equally important opportunities for HIV prevention. For example, evidence exists for a cofactor role of vaginitis caused by the common, sexually transmitted parasite T. vaginalis, so including T. vaginalis in screening protocols for women whenever feasible is likely to lower the risk for HIV transmission. Infections with HSV-2 are highly prevalent in the U.S. population (45) and occur in at least half of individuals in some subpopulations at high risk for HIV infection (e.g., MSM). As a persistent, latent infection causing recurring genital ulcers and associated with greater genital tract HIV shedding (16), HSV-2 coinfection could represent a major STD cofactor effect. At a minimum, persons with both HIV infection and genital herpes should be counseled especially to avoid sex when herpes is symptomatic because HIV viral shedding is more active during such periods. However, the optimal detection and treatment strategy corresponding to this particular STD/HIV interaction has not been well-defined and, for the moment, remains a critical area for needed research. Emerging data on bacterial vaginosis as a risk factor for HIV acquisition in women (13,102) likewise represent a major potential opportunity, as well as a challenge. Although bacterial vaginosis is a highly prevalent condition, no well-defined effective strategies for long-term prevention exist beyond treating individual patients. RESEARCH ISSUES In addition to practical steps that can be implemented immediately, several research issues need to be addressed to maximize the longer-term impact of early STD detection and treatment as a strategy for HIV prevention. These include a) methods to better assess, at national and local levels, the attributable risks related to different STD pathogens; b) methods to better assess the potential prevention impact of different approaches to enhanced STD treatment and prevention; c) protocols for assessing access to and quality of clinical STD services in communities and for specific populations within communities; d) the prevalence of STDs and risk factors for STDs among asymptomatic persons currently not screened for STDs (e.g., men), so screening guidelines can be refined further; e) the incidence of curable STDs in certain high-risk populations (e.g., HIV-infected persons), so guidelines can better specify the best frequency of screening; f) the field performance and practical issues involved in using both new and older noninvasive tests (e.g., urine tests and self-obtained swabs) to identify STDs in nonmedical settings; g) the precise balance of benefits and risks of presumptive or prophylactic antimicrobial therapy for persons who, based on epidemiologic data, have extremely high rates of curable STDs; and h) the role of HSV-2 and other viral STDs in HIV transmission and the potential role of suppressive or other chemotherapy for genital herpes in reducing the risk for HIV transmission. Similarly, new behavioral and operational research is required to complement, facilitate, and enhance overall HIV prevention efforts because of increased emphasis on early STD detection and treatment. Examples include how to best provide HIV and STD prevention counseling when asymptomatic STDs are detected and how to improve HIV prevention referral systems and processes within the full range of STD detection and treatment facilities and settings (i.e., HIV counseling and testing centers, MCOs, and street outreach settings). Operations research also is needed to better understand how to organize STD prevention services to prevent HIV transmission. Additional research issues will arise as the initial program activities described previously are implemented. CONCLUSIONS Early detection and treatment of other STDs should be a critical component of national, state, and local strategies to prevent HIV infection and AIDS, in concert with the behavioral and other interventions that constitute a comprehensive HIV prevention approach. Because the United States has the highest rates of curable STDs among industrialized nations (39,40) and a high prevalence of HIV infection (103), the potential impact of enhanced STD control on the prevention of sexually transmitted HIV infections in the United States is likely to be substantial. The initial steps outlined in this report should be implemented by state and local HIV and STD prevention programs as part of a comprehensive HIV prevention effort. Evaluation of these initial efforts should be used to guide subsequent implementation of this strategy. Acknowledgment The authors acknowledge the substantive contributions of Thomas A. Farley, M.D., M.P.H., Louisiana Office of Public Health, and Deborah A. Cohen, M.D., M.P.H., Louisiana State University. References
Figure_1 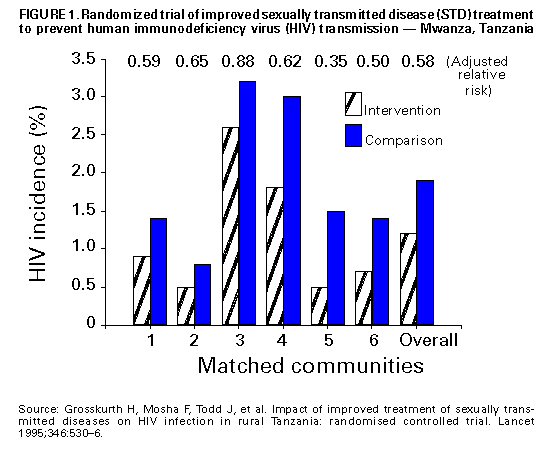 Return to top. Figure_2 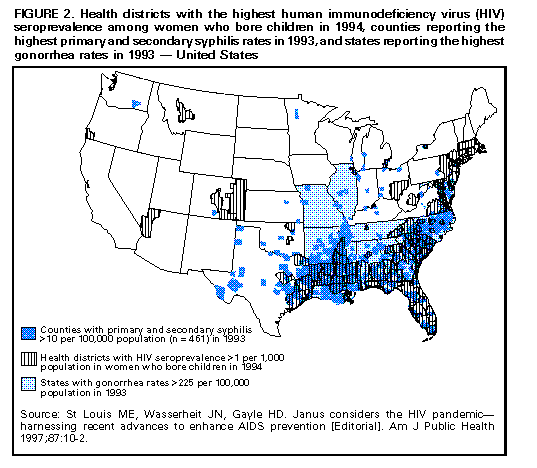 Return to top. Figure_3 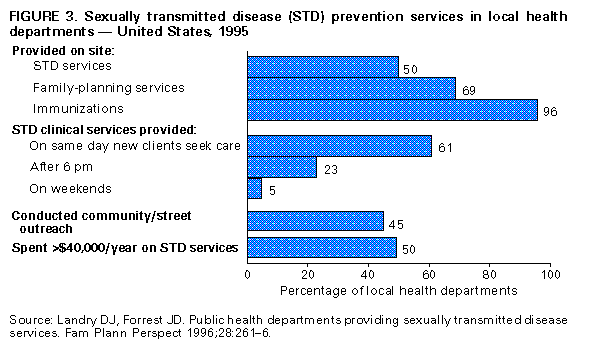 Return to top. Disclaimer All MMWR HTML versions of articles are electronic conversions from ASCII text into HTML. This conversion may have resulted in character translation or format errors in the HTML version. Users should not rely on this HTML document, but are referred to the electronic PDF version and/or the original MMWR paper copy for the official text, figures, and tables. An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S. Government Printing Office (GPO), Washington, DC 20402-9371; telephone: (202) 512-1800. Contact GPO for current prices. **Questions or messages regarding errors in formatting should be addressed to mmwrq@cdc.gov.Page converted: 10/05/98 |
|||||||||
This page last reviewed 5/2/01
|