 |
|
|
|
|
|
|
| ||||||||||
|
|
|
|
|
|
|
||||
| ||||||||||
|
|
|
|
|
Persons using assistive technology might not be able to fully access information in this file. For assistance, please send e-mail to: mmwrq@cdc.gov. Type 508 Accommodation and the title of the report in the subject line of e-mail. Community Outbreak of Hemolytic Uremic Syndrome Attributable to Escherichia coli O111:NM -- South Australia, 1995Postdiarrheal hemolytic uremic syndrome (HUS) is characterized by microangiopathic hemolytic anemia, renal injury, and thrombocytopenia and is associated with infection with Shiga-like toxin-producing Escherichia coli (SLTEC). From January 4 through February 20, 1995, the South Australian Communicable Disease Control Unit of the Health Commission (SACDCU) received reports of 23 cases of HUS among children aged less than 16 years who resided in South Australia. In comparison, during 1994, a total of three cases of HUS was reported in South Australia (1991 population: 1.4 million). This report summarizes preliminary findings of the investigation of this outbreak by SACDCU, Women's and Children's Hospital, Institute of Medical and Veterinary Science, and the National Center for Epidemiology and Population Health of Australian National University. Three cases of HUS were reported to SACDCU during January 4-16. Subsequently, SACDCU requested that hospitals, commercial clinical laboratories, general practitioners, and -- with the cooperation of the news media -- the public throughout South Australia report persons with bloody diarrhea, HUS, or thrombotic thrombocytopenic purpura (TTP). The preliminary investigation suggested that HUS occurred as a complication of infection associated with consumption of uncooked, semi-dry fermented sausage product produced locally by a single manufacturer. On January 23, the South Australian Health Commission issued a press release noting the link to the sausage; the manufacturer subsequently initiated a recall Figure_1 of products with a "use by" date of March 12, later extended to include products with dates during January 26-April 12. The median age of the 23 patients with HUS was 4 years (range: 4 months-12 years); 14 (61%) were male. Most (19 {83%}) patients resided in the city of Adelaide, and four resided in surrounding rural areas. Sixteen (70%) patients required dialysis; one 4-year-old girl died. Twenty-two of the patients had had onset of diarrhea during the 2 weeks preceding the diagnosis of HUS; of these, 16 had bloody diarrhea. During the 8 days preceding onset of illness, 16 patients had consumed uncooked, semi-dry fermented sausage produced locally by a single manufacturer; for three other patients, this product recently had been kept in the household, although consumption by the patients was not confirmed. Stool specimens obtained from all 23 patients during their illness were screened using polymerase chain reaction (PCR) for the genes encoding for Shiga-like toxins (SLTs) I and II (1); of these, 20 (87%) were positive for both SLTs I and II, one (4%) was positive for only SLT II, and two (9%) were negative. E. coli O111:NM (nonmotile) subsequently was isolated from stool specimens from 16 of these patients. Other E. coli strains positive by PCR for SLT also were detected in specimens from three patients. In addition to the 23 cases of HUS, physicians reported 30 persons with bloody diarrhea from whom no other bacterial pathogens had been isolated and three adults with TTP. Stool samples from eight (24%) of these 33 persons were PCR-positive for SLT genes, but E. coli O111:NM was isolated from only one. SACDCU also received 105 reports of persons with gastrointestinal illness other than bloody diarrhea; 32 (30%) had a history of consumption of the implicated sausage. Stool specimens from 20 of these persons were positive for SLT by PCR. SLTEC were isolated from all 20 of these PCR-positive specimens, and isolates from two persons were identified as E. coli O111:NM. Of 10 sausage samples taken during January 19-February 8 from the homes of nine patients (eight homes total), eight (all from the same manufacturer) were positive for SLTs I and II by PCR; E. coli O111:NM was isolated from four of these samples. Eighteen (39%) of 47 additional sausage samples produced by the same manufacturer obtained during January 19-March 9 from homes where diarrheal illness without HUS occurred and from retail stores were PCR positive; three yielded E. coli O111:NM. Sixty-three samples of sausage from other manufacturers were collected during the same period from retail outlets and from homes of persons with diarrheal illness but not HUS; E. coli O111:NM was not isolated from any of these specimens. Industry and food agencies in South Australia, in conjunction with the National Food Authority and the Department of Primary Industry and Energy, are investigating the implicated products and the quality controls employed by the manufacturer and its suppliers to determine the specific source of contamination. In addition, comparative epidemiologic studies are ongoing. Reported by: AS Cameron, MD, MY Beers, CC Walker, N Rose, E Anear, Z Manatakis, K Kirke, MBBS, I Calder, PhD, F Jenkins, PhD, Public and Environmental Health Svc, South Australian Health Commission; PN Goldwater, MBBS, A Paton, PhD, J Paton, PhD, K Jureidini, MBBS, A Hoffman, P Henning, MBBS, D Hansman, MBBS, A Lawrence, MSc, R Miller, Women's and Children's Hospital, Adelaide, South Australia; R Ratcliff, R Doyle, C Murray, D Davos, P Cameron, J Seymour-Murray, I Lim, MBBS, J Lanser, PhD, Institute of Medical and Veterinary Science, Adelaide, South Australia; L Selvey, PhD, S Beaton, National Center for Epidemiology and Population Health, Australian National Univ, Canberra, Australia. Foodborne and Diarrheal Diseases Br, Div of Bacterial and Mycotic Diseases, National Center for Infectious Diseases, CDC. Editorial NoteEditorial Note: SLTEC are now recognized as a cause of postdiarrheal HUS and TTP. Based on studies in North America and the United Kingdom, antecedent infection with one serogroup -- E. coli O157 -- may account for greater than 75% of cases of postdiarrheal HUS in these locations (2,3). In addition, however, greater than 100 non-O157 SLTEC serotypes have been isolated from humans; most of these serotypes have been isolated from persons with HUS (3). This report documents the second outbreak of a non-O157 SLTEC with a probable link to a food product (4), and follows the recent report of an E. coli O157:H7 outbreak associated with a similar dry fermented sausage product in the United States (5). In Australia, E. coli O157 has not been isolated frequently; among non-O157 SLTEC, E. coli O111 is common. At one laboratory during 1987-1994, seven (50%) of 14 non-O157 SLTEC strains from persons with HUS in Australia identified were E. coli O111 (6). Outbreaks attributable to non-O157 SLTEC rarely have been reported. In an outbreak of SLTEC O111 infections in Italy during 1992, all nine patients had HUS, but a common source was not identified (7). In Australia, two cases of HUS attributable to O111 infection were reported in siblings residing in the same household (8). The outbreak described in this report is the largest reported community outbreak of HUS associated with E. coli O111 infection. In June 1994, HUS in persons aged less than 16 years became notifiable to the Australian Pediatric Surveillance Unit of the Australian College of Pediatrics. Reports of HUS are transmitted from participating pediatric microbiologists and nephrologists to the surveillance unit. Prompt reporting of HUS was important in recognizing this outbreak, determining the responsible pathogen, and removing the suspected source from the market to prevent additional cases. Based on an experimental inoculation study, E. coli O157:H7 survives the fermentation and drying process used in preparing products similar to those in this report (9). Isolation of E. coli O111 from dried sausage, in combination with the finding that non-O157 SLTEC commonly are isolated from the intestines of food animals (10), suggests that control measures for E. coli O157:H7 also can prevent E. coli O111 infections. These recommendations include the need to avoid eating raw or undercooked ground meats and prevent cross-contamination in the kitchen, and to wash hands, utensils, and preparation surfaces that have come in contact with raw meat. In general, children with any acute diarrheal illness should be excluded from child day care centers; children aged less than 5 years infected with SLTEC should not return to child day care centers until they are asymptomatic and have had two negative stool cultures. In addition, food handlers and health-care workers infected with SLTEC should not return to work until they are asymptomatic and have had two negative stool cultures. The E. coli O111 strain associated with the outbreak in this report ferments sorbitol -- a characteristic that distinguishes this strain from E. coli O157:H7. In this outbreak, E. coli O111 would not have been detected by sorbitol-MacConkey medium, which is recommended for screening for E. coli O157:H7. Instead, screening by PCR coupled with serotyping of E. coli from PCR-positive specimens enabled detection of the pathogen in stool specimens and epidemiologically related food. Non-O157 SLTEC can be detected by screening stool specimens for SLTEC with PCR or genetic probes. However, such methods generally are not available for clinical laboratories. Therefore, in the United States, health-care providers who identify clusters of persons with bloody diarrhea or HUS from whom stool cultures do not yield E. coli O157:H7 should request that state health departments examine specimens for other SLTEC. In suspected cases, frozen stool specimens and isolates from routine culture plates can be saved for examination. References
Figure_1 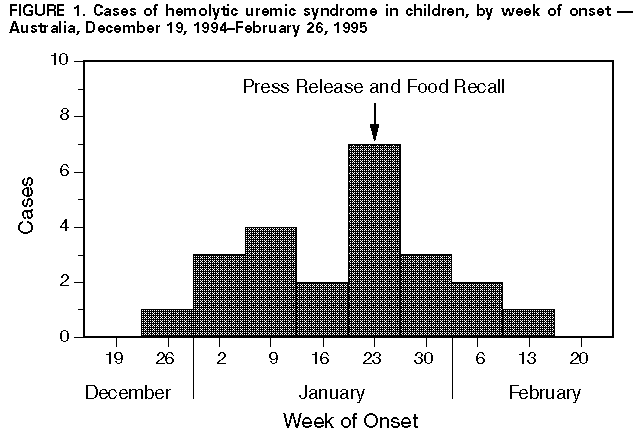 Return to top. Disclaimer All MMWR HTML versions of articles are electronic conversions from ASCII text into HTML. This conversion may have resulted in character translation or format errors in the HTML version. Users should not rely on this HTML document, but are referred to the electronic PDF version and/or the original MMWR paper copy for the official text, figures, and tables. An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S. Government Printing Office (GPO), Washington, DC 20402-9371; telephone: (202) 512-1800. Contact GPO for current prices. **Questions or messages regarding errors in formatting should be addressed to mmwrq@cdc.gov.Page converted: 09/19/98 |
|||||||||
This page last reviewed 5/2/01
|