 |
|
|
|
|
|
|
| ||||||||||
|
|
|
|
|
|
|
||||
| ||||||||||
|
|
|
|
|
Persons using assistive technology might not be able to fully access information in this file. For assistance, please send e-mail to: mmwrq@cdc.gov. Type 508 Accommodation and the title of the report in the subject line of e-mail. Pertussis -- United States, January 1992-June 1995Pertussis was a major cause of morbidity and mortality among infants and children in the United States during the prevaccine era (i.e., before the mid-1940s). Since pertussis became a nationally reportable disease in 1922, the highest number of pertussis cases (approximately 260,000) was reported in 1934; the highest number of pertussis-related deaths (approximately 9000) occurred in 1923. Following the licensure of whole-cell pertussis vaccine combined with diphtheria and tetanus toxoids (DTP) in 1949 and the widespread use of DTP among infants and children, the incidence of reported pertussis declined to a historical low of 1010 cases in 1976 Figure_1. However, since the early 1980s, reported pertussis incidence has increased cyclically with peaks occurring in 1983, 1986, 1990, and 1993 (1-3). This report summarizes national surveillance data for pertussis from January 1992 through June 1995 from CDC's National Public Health Surveillance System (NPHSS) and Supplementary Pertussis Surveillance System (SPSS) and assesses the effectiveness of pertussis vaccination in the United States during this period using vaccination coverage data from CDC's National Health Interview Survey (NHIS). National Surveillance for Pertussis and Vaccination Coverage Through NPHSS (formerly the National Notifiable Disease Surveillance System), state health departments report weekly to CDC the number of pertussis cases. Data reported include state and county of residence, age, date of report to CDC, and race/ethnicity. Through SPSS, more detailed information about persons with pertussis is reported to CDC, including demographic variables, vaccination history, selected clinical characteristics, hospital admission, deaths, and results of laboratory tests for Bordetella pertussis. Documented limitations of these pertussis surveillance systems include underreporting, disproportionate representation of classic and severe cases, lack of uniform reporting criteria among the states, and reliance on laboratory diagnosis of pertussis by some states (1). NHIS is an annual cross-sectional household interview survey of the U.S. civilian, noninstitutionalized population (4). In 1992, an immunization supplement was added to the survey to collect data about vaccinations among children aged less than 6 years. Vaccination information was obtained from vaccination records; for children for whom no vaccination records were available (50%-65%), information was based on parental recall. Based on NPHSS data, from 1992 through 1994, a total of 15,286 pertussis cases were reported to CDC (4083 in 1992; 6586 in 1993; and 4617 in 1994), for crude annual incidence rates of 1.6, 2.6, and 1.8 cases per 100,000 population in 1992, 1993, and 1994, respectively. Cases were reported from all 50 states and the District of Columbia. From January 7 through June 30, 1995, a total of 1386 pertussis cases were reported -- an 18% decrease from the number reported during the same period in 1994 (1690). Based on the NPHSS, during 1992-1994, of 13,615 persons reported with pertussis for whom age data were available, 5618 (41%) were aged less than 1 year; 2682 (20%), 1-4 years; 1551 (11%), 5-9 years; and 3764 (28%), greater than or equal to 10 years. Of the children aged less than 1 year with pertussis, 4524 (81%) were aged less than 6 months. Of 10,989 patients for whom data about vaccination status were available from SPSS, 6876 (63%) had received fewer than three doses of DTP. Of 3184 patients aged 7 months-4 years for whom vaccination status was known, 725 (23%) had received no doses, 714 (22%) had received one or two doses, and 1745 (55%) had received three or more doses. The proportion of patients who were hospitalized, had complications, or died was highest among infants and decreased with increasing age Table_1. Of children aged less than 1 year reported with pertussis, 66% were hospitalized, 15% had pneumonia confirmed radiographically, and 2% had seizures. Overall, 32 pertussis- related deaths and 17 cases complicated by encephalopathy were reported. Based on the NHIS, from 1992 through the second quarter of 1994 (the most recent period for which data were available), among children aged 19-35 months (median age: 27 months), vaccination coverage with three or more doses of DTP or diphtheria and tetanus toxoids (DT) was 83% for 1992, 88% for 1993, 87% for the first quarter of 1994, and 90% for the second quarter. Vaccination coverage with four or more doses of DTP or DT was 59% in 1992, 72% for 1993, 67% for the first quarter of 1994, and 70% for the second quarter. Based on vaccine distribution data for 1993, 6.7% of children may have received DT instead of DTP (CDC, unpublished data, 1993). Effectiveness of Pertussis Vaccination The screening method (5) was used to calculate the effectiveness of pertussis vaccine among U.S. children aged 7-47 months during 1992-1994. Estimates of vaccine effectiveness (VE) were derived using the formula VE=1-{PCV/(1-PCV)}{(1-PPV)/PPV} (PPV is the proportion of the population vaccinated, and PCV is the proportion of case-patients vaccinated). Persons who were partially vaccinated (i.e., received one to two doses of vaccine) were excluded from both PPV and PCV. Data from the national SPSS were used to determine the PCV. A case of pertussis was defined as either onset of a cough illness of any duration with isolation of B. pertussis from a clinical specimen or onset of an acute cough illness lasting greater than or equal to 14 days plus at least one pertussis-associated symptom (i.e., paroxysms of cough, inspiratory "whoop," or posttussive vomiting) with no other apparent cause. Data from NHIS for 1992, 1993, and the first 2 quarters of 1994 were used to determine PPV for age groups 7-18 months and 19-47 months. Compared with zero doses of pertussis vaccine, during 1992-1994, among children aged 7-18 months, VE for three doses was 85%; among children aged 19-47 months, VE for four or more doses was 94%. When these estimates were corrected by 6.7% to account for use of DT instead of DTP, VE was 64% and 82% for three doses and four or more doses, respectively. Reported by: State and local health depts. Child Vaccine Preventable Disease Br, Epidemiology and Surveillance Div, and Assessment Br, Data Management Div, National Immunization Program, CDC. Editorial NoteEditorial Note: Despite the upward trend in the reported incidence of pertussis in the United States since the early 1980s, the annual numbers of cases reported during 1992-1994 represent an approximately 95% decline from those reported during the prevaccine era. Following the peak in reported cases in 1993, the numbers declined during 1994 and the first 2 quarters of 1995 -- a pattern consistent with the previously observed 3-4-year periodicity in pertussis incidence. Pertussis remains an important cause of morbidity and mortality among infants and preschool-aged children. Rates of complications among infants during 1992-1994 are similar to those reported during 1980-1989 (1) and 1989-1991 (2). The two groups at greatest risk for severe complications are infants aged less than 6 months (the recommended age by which children should have received three doses of DTP) and preschool-aged children who are undervaccinated. The importance of timely vaccination of children is emphasized by the high proportion of undervaccination (approximately 45%) among preschool-aged children with pertussis who were age-eligible for at least three doses of vaccine. The Advisory Committee on Immunization Practices and the American Academy of Pediatrics recommend three doses of DTP to be administered at ages 2, 4, and 6 months. An additional two doses are recommended, one each at ages 12-18 months and at 4-6 years (6). Either DTP or diphtheria and tetanus toxoids and acellular pertussis vaccine (DTaP) can be administered for the fourth and fifth doses to children aged 15 months-6 years. Since 1992, coverage with three doses of DTP or DT has increased, indicating progress toward the Childhood Immunization Initiative goal of 90% coverage by 1996. As a consequence, the proportion of persons with pertussis who have been vaccinated most likely will increase. Based on the screening method (which accounts for changes in vaccination coverage but may not provide an accurate estimate of vaccine efficacy when vaccination coverage is high) (5), estimated VE during 1992-1994 was consistent with previous reports about the efficacy of whole-cell pertussis vaccine in the United States during the mid-1980s, which documented 64% protection against mild disease and 95% protection against severe disease (7). In the United States, widespread use of whole-cell pertussis vaccines among infants since 1949 has resulted in the successful control of pertussis. National pertussis surveillance data during January 1992-June 1995 indicate the continued effectiveness of the current pertussis vaccination program. However, despite increasing vaccination coverage in recent years, pertussis outbreaks (e.g., in Cincinnati and Chicago in 1993 {3}) continue to occur. Preliminary results of the protective efficacy of new acellular pertussis vaccines (when used for the first three doses among infants) suggest that these vaccines are either equally or more efficacious than whole-cell vaccines. Further scientific review of these results is in progress, but until such vaccines are licensed and available for use among infants, timely age-appropriate vaccination of infants with whole-cell pertussis vaccines should continue. Previous delays in administering pertussis vaccine to infants have resulted in widespread outbreaks (e.g., in the United Kingdom and Japan during the 1970s and Sweden during the 1980s) (8). References
Figure_1 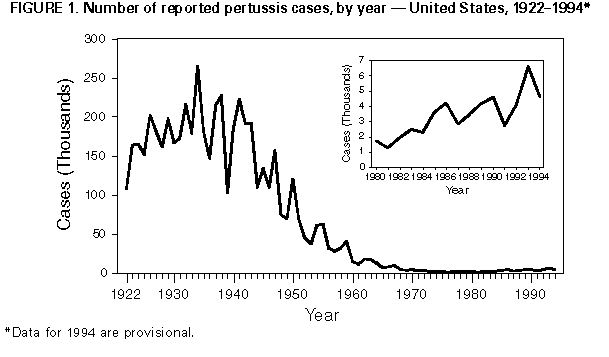 Return to top. Table_1 Note: To print large tables and graphs users may have to change their printer settings to landscape and use a small font size.
TABLE 1. Number of pertussis-related hospitalizations, complications, and deaths, by age
group -- United States, 1992-1994
===============================================================================================
Complications
------------------------------------
Encephalo-
Hospitalized Pneumonia * Seizures pathy Deaths
No. persons ------------ ----------- --------- ----------- --------------
Age group with pertussis No. (%) No. (%) No. (%) No. (%) No. (%)
-----------------------------------------------------------------------------------------------
<6 mos 4,524 3,217 (71.1) 671 (14.8) 87 (1.9) 11 ( 0.2) 25 ( 0.6)
6-11 mos 1,094 512 (46.8) 153 (14.0) 27 (2.5) 2 ( 0.2) 3 ( 0.3)
1- 4 yrs 2,682 580 (21.6) 248 ( 9.2) 45 (1.7) 3 ( 0.1) 1 (<0.1)
5- 9 yrs 1,551 124 ( 8.0) 66 ( 4.3) 8 (0.5) 0 3 ( 0.2)
10-19 yrs 2,223 78 ( 3.5) 45 ( 2.0) 10 (0.4) 1 (<0.1) 0
>=20 yrs 1,541 57 ( 3.7) 41 ( 2.7) 7 (0.5) 0 0
Total 13,615 + 4,568 & (33.6) 1,224 @ ( 9.0) 184 (1.4) 17 ( 0.1) 32 ( 0.2)
-----------------------------------------------------------------------------------------------
* Radiographically confirmed
+ Excludes 19 (0.1%) patients of unknown age with pertussis.
& Excludes six hospitalized patients of unknown age.
@ Excludes one hospitalized patient of unknown age.
===============================================================================================
Return to top. Disclaimer All MMWR HTML versions of articles are electronic conversions from ASCII text into HTML. This conversion may have resulted in character translation or format errors in the HTML version. Users should not rely on this HTML document, but are referred to the electronic PDF version and/or the original MMWR paper copy for the official text, figures, and tables. An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S. Government Printing Office (GPO), Washington, DC 20402-9371; telephone: (202) 512-1800. Contact GPO for current prices. **Questions or messages regarding errors in formatting should be addressed to mmwrq@cdc.gov.Page converted: 09/19/98 |
|||||||||
This page last reviewed 5/2/01
|