 |
|
|
|
|
|
|
| ||||||||||
|
|
|
|
|
|
|
||||
| ||||||||||
|
|
|
|
|
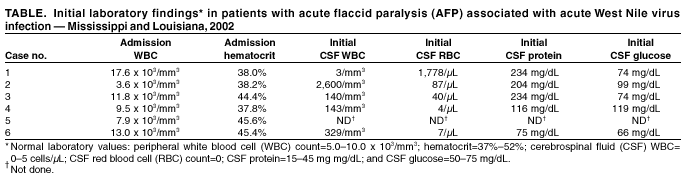
Persons using assistive technology might not be able to fully access information in this file. For assistance, please send e-mail to: mmwrq@cdc.gov. Type 508 Accommodation and the title of the report in the subject line of e-mail. Acute Flaccid Paralysis Syndrome Associated with West Nile Virus Infection --- Mississippi and Louisiana, July--August 2002West Nile virus (WNV) infection can cause severe, potentially fatal neurologic illnesses including encephalitis and meningitis (1,2). Acute WNV infection also has been associated with acute flaccid paralysis (AFP) attributed to a peripheral demyelinating process (Guillain-Barré Syndrome [GBS]) (3), or to an anterior myelitis (4). However, the exact etiology of AFP has not been assessed thoroughly with electrophysiologic, laboratory, and neuroimaging data. This report describes six cases of WNV-associated AFP in which clinical and electrophysiologic findings suggest a pathologic process involving anterior horn cells and motor axons similar to that seen in acute poliomyelitis. Clinicians should evaluate patients with AFP for evidence of WNV infection and conduct tests to differentiate GBS from other causes of AFP. Case ReportsCase 1. In July 2002, a previously healthy man aged 56 years from Mississippi was admitted to a local hospital with a 3-day history of fever, chills, vomiting, confusion, and acute painless weakness of the arms and legs. On physical examination, he had tremor and areflexic weakness in both arms and asymmetric weakness in the legs with hypoactive reflexes; sensation was intact. Laboratory abnormalities included a mildly elevated protein in the cerebrospinal fluid (CSF) (Table). An evolving stroke was diagnosed, and the patient was treated with anticoagulant therapy; subsequently, the illness was attributed to GBS, and intravenous immune globulin (IVIG) therapy was initiated. A computerized tomography (CT) scan and magnetic resonance imaging (MRI) of the brain and cervical spine were normal. Electromyography and nerve-conduction studies (EMG/NCS) were indicative of a severe asymmetric process involving anterior horn cells and/or their axons. An acute WNV infection was considered probable on the basis of the presence of virus-specific IgM antibody in serum. Case 2. In July 2002, a man aged 57 years from Mississippi was admitted to a local hospital with a 3-day history of fever, chills, vomiting, and headache. Laboratory abnormalities indicated an elevated protein and pleocytosis in the CSF (Table). The patient subsequently had acute respiratory failure requiring intubation. On physical examination, rigidity in all extremities was observed with no spontaneous movement. Following extubation, bilateral facial and areflexic, asymmetric weakness was observed in all extremities; sensory examination was normal. Brain MRI was normal. EMG/NCS were indicative of a severe asymmetric process affecting anterior horn cells and/or their axons. IgM and neutralizing antibody test results confirmed an acute WNV infection. Case 3. In July 2002, a man aged 56 years from Louisiana with a history of hypertension and coronary artery disease was hospitalized with a 4-day history of fever, vomiting, and painless asymmetric leg weakness. On examination, the patient had a flaccid areflexic right leg and a weak, hyporeflexic left leg; strength and reflexes in the arms were normal. The patient had decreased sensation in a stocking-and-glove distribution and a coarse upper extremity action tremor. A lumbar puncture revealed a CSF pleocytosis (Table). He was admitted with a diagnosis of postviral demyelination syndrome and treated with antimicrobial medication, IVIG, and dexamethasone. MRI of the spine revealed mild cervical spinal stenosis and homogeneous enhancement of the cauda equina consistent with meningitis. EMG/NCS were indicative of a severe asymmetric process affecting anterior horn cells and/or their axons. IgM and neutralizing antibody test results confirmed an acute WNV infection. Case 4. In August 2002, a woman aged 69 years from Louisiana with a history of diabetes and degenerative disc disease was hospitalized with a 1-day history of vomiting, lethargy, confusion, fever, and painless right arm weakness. On examination, the patient had nuchal rigidity and a coarse tremor in the chin, left arm, and both legs. The right arm was flaccid and areflexic; strength and reflexes in the other extremities were normal. She was admitted with a diagnosis of meningoencephalitis with associated focal motor radiculitis versus monoplegia secondary to cerebrovascular ischemia. Head CT and brain MRI showed chronic microvascular ischemic changes; MRI of the cervical spine displayed mild narrowing of the spinal cord and the right neural foramina at the C5-6 level. EMG/NCS indicated a severe, asymmetric process affecting anterior horn cells and/or their axons. IgM and neutralizing antibody test results confirmed an acute WNV infection. Case 5. In August 2002, a previously healthy man aged 50 years from Mississippi was hospitalized with vomiting and headache. He had flaccid, areflexic weakness in the right arm; sensation in all extremities was normal. An acute stroke was diagnosed, and the patient received anticoagulant therapy. EMG/NCS were indicative of a severe, asymmetric process affecting anterior horn cells and/or their axons in the right upper extremity. IgM and neutralizing antibody test results confirmed an acute WNV infection. Case 6. In August 2002, a man aged 46 years from Louisiana with a history of coronary artery disease was admitted to a hospital with fever, chills, fatigue, and leg weakness. He had a plegic and areflexic right leg and mild left leg weakness; sensation was intact. Laboratory abnormalities included a lymphocytic pleocytosis in CSF (Table). The patient was admitted with a diagnosis of GBS and treated with IVIG and antibiotics. An enhanced MRI of the spine revealed findings suggestive of meningitis involving the conus medullaris and cauda equina. EMG/NCS indicated a severe, asymmetric process affecting anterior horn cells and/or their axons. IgM and neutralizing antibody test results confirmed an acute WNV infection. Reported by: A Leis, MD, D Stokic, MD, J Polk, MD, V Dostrow, MD, M Winkelman, MD, Methodist Rehabilitation Center, Jackson; R Webb, MD, S Slavinski, DVM, M Currier, MD, State Epidemiologist, Mississippi State Dept of Health. J Van Gerpen, MD, Ochsner Clinic, New Orleans; E Brewer, MD, R Ratard, MD, State Epidemiologist, Louisiana Office of Public Health. J Sejvar, MD, Div of Viral and Rickettsial Diseases; L Petersen, MD, A Marfin, MD, G Campbell, MD, Div of Vector-Borne Infectious Diseases, National Center for Infectious Diseases; B Tierney, MD, M Haddad, MSN, S Montgomery, DVM, A Vicari, DVM, EIS officers, CDC. Editorial Note:The clinical, laboratory, and electrophysiologic findings of these six patients suggest that WNV-associated AFP is a polio-like syndrome with involvement of the anterior horn cells of the spinal cord and motor axons. All six patients had acute onset of asymmetrical weakness without pain or sensory loss. All but one of those with CSF drawn had pleocytosis. Investigation of these patients is continuing. A polio-like syndrome has been associated with flaviviruses other than WNV (5), and anterior myelitis has occurred with WNV infection (4). Investigations of primates (6) and other vertebrates infected with WNV have documented involvement of spinal motor neurons and lesions in the ventral gray matter of the spinal cord, with an absence of lesions in peripheral nerves. Previous case series have attributed WNV infection--associated AFP to a peripheral neuronal process similar to GBS; acute poliomyelitis might simulate GBS, causing diagnostic confusion (7). Clinical, laboratory, and electrophysiologic features of these cases might help differentiate poliomyelitis from GBS. In comparison with the asymmetric AFP observed in these patients, GBS syndrome is more often symmetric, generally involves sensory changes or paresthesias, and is associated with an elevation of CSF protein in the absence of pleocytosis. Additional features of typical GBS include an onset several days following signs of acute infection and a generally favorable outcome with rapid improvement in strength. In addition, EMG/NCS typically suggest a predominantly demyelinating picture, or a combined axonal and demyelinating process. A pure motor axonal variant of GBS (8) might be confused with polio; however, this GBS variant is typically characterized by symmetric, distally prominent weakness and subclinical sensory nerve involvement on EMG/NCS. Treatment modalities used for patients with GBS include anticoagulation, IVIG, plasmapheresis, and high-dose corticosteroids. These therapies have no beneficial effect for poliomyelitis and can have significant morbidity (9,10). In areas where WNV transmission is occurring, clinicians should suspect acute WNV infection and conduct appropriate diagnostic tests in patients presenting with acute, painless, asymmetric weakness, particularly in the setting of an acute febrile illness with CSF pleocytosis. In addition, CSF analysis, thorough EMG/NCS, and neuroimaging should be strongly considered before initiating therapies for GBS or other peripheral inflammatory processes. Continued surveillance and public health investigation is needed to fully define the scope of neurologic illnesses associated with WNV infection. Health-care providers who are aware of patients with acute WNV infection and AFP should contact their state or local health departments and CDC, telephone 404-639-4657; e-mail, zea3@cdc.gov. References
Acknowledgments This report is based on information contributed by S Kemmerly, MD, K Baumgarten, MD, Ochsner Clinic; M Rosenblum, MD, K Landry, Touro Infirmary, New Orleans; P Vaccaro, P Mussarat, MD, North Oaks Hospital, Hammond; J Lefran, MD, G Reddy, MD, T Croney, Slidell Memorial Hospital, Slidell, Louisiana. P Collins, Div of Vector-Borne Infectious Diseases, National Center for Infectious Diseases, CDC.
 Return to top. |
Use of trade names and commercial sources is for identification only and does not imply endorsement by the U.S. Department of
Health and Human Services. |
Disclaimer All MMWR HTML versions of articles are electronic conversions from ASCII text into HTML. This conversion may have resulted in character translation or format errors in the HTML version. Users should not rely on this HTML document, but are referred to the electronic PDF version and/or the original MMWR paper copy for the official text, figures, and tables. An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S. Government Printing Office (GPO), Washington, DC 20402-9371; telephone: (202) 512-1800. Contact GPO for current prices.
**Questions or messages regarding errors in formatting should be addressed to mmwrq@cdc.gov.Page converted: 9/19/2002
|
|
|||||
|
HOME |
ABOUT MMWR |
MMWR SEARCH |
DOWNLOADS |
RSS
|
CONTACT
|
|||||
|
|
|||||
|
This page last reviewed 9/19/2002