 |
|
|
|
|
|
|
| ||||||||||
|
|
|
|
|
|
|
||||
| ||||||||||
|
|
|
|
|
Persons using assistive technology might not be able to fully access information in this file. For assistance, please send e-mail to: mmwrq@cdc.gov. Type 508 Accommodation and the title of the report in the subject line of e-mail. Update: Investigation of Bioterrorism-Related Anthrax and Interim Guidelines for Clinical Evaluation of Persons with Possible AnthraxSince October 3, 2001, CDC and state and local public health authorities have been investigating cases of bioterrorism-related anthrax. This report updates findings as of October 31, and includes interim guidelines for the clinical evaluation of persons with possible anthrax. A total of 21 cases (16 confirmed and five suspected) of bioterrorism-related anthrax have been reported among persons who worked in the District of Columbia, Florida, New Jersey, and New York City (Figure 1). Until the source of these intentional exposures is eliminated, clinicians and laboratorians should be alert for clinical evidence of Bacillus anthracis infection. Epidemiologic investigation of these cases and surveillance to detect new cases of bioterrorism-associated anthrax continues. New YorkTo date, the investigations in New York City have identified one confirmed inhalational case and six (three confirmed and three suspected) cutaneous anthrax cases; the confirmed inhalational and one suspected cutaneous case have been identified since the last report (1). The six cutaneous cases were associated with four media companies (A--D); the most recent suspected cutaneous case is associated with company D. The most recent confirmed inhalational case is not directly associated with any media company or with mail handling. No cases among postal workers have been identified. The most recent suspected cutaneous case occurred in a 34-year-old man who worked in the mail room of company D who might have handled a letter postmarked September 18, which the patient handled during October 12--15 and subsequently was found to contain B. anthracis (1). On October 19, the patient noted a small, erythematous pruritic papule on his left forearm that later developed a small vesicle. On October 21, he started ciprofloxacin. By October 22, an eschar had developed, increased in size, and over the next several days was surrounded by erythema, edema, and induration. A biopsy was positive for B. anthracis by immunohistochemical (IHC) staining. The inhalational anthrax case occurred in a 61-year-old woman who worked in the stockroom of a hospital in Manhattan. The patient became ill on October 25 with malaise and myalgias. During the next several days, she had shortness of breath, chest discomfort, and a productive cough with blood-tinged sputum. She reported no fever, chills, or night sweats. She presented to an emergency department on October 28 in respiratory distress. Her temperature was 102 F (39 C), and she was admitted to the intensive care unit and required mechanical ventilation. Initial chest radiograph revealed pulmonary venous congestion and bilateral pleural effusions; a chest computerized tomography (CT) scan revealed a widened mediastinum and bilateral pleural effusions. An echocardiogram indicated a small pericardial effusion. She was empirically treated with levofloxacin, rifampin, and clindamycin. Blood cultures grew B. anthracis less than 24 hours after admission. Her pleural effusion revealed hemorrhagic fluid and B. anthracis. The patient died on October 31. New JerseyTo date, investigations in New Jersey and Pennsylvania have identified seven (five confirmed and two suspected) anthrax cases. Since the last report (1), cutaneous disease was confirmed in two patients, and inhalational anthrax was confirmed in two patients, one of whom was previously classified as a suspected case-patient. Five patients worked in New Jersey at one of two postal facilities. Although no specific contaminated letter was implicated in these cases, contaminated letters destined for both New York City and the District of Columbia passed through at least one of the postal facilities in New Jersey. Inhalational anthrax was confirmed in a 56-year-old female postal worker who initially was classified as a suspected case-patient (1). Her pleural fluid was positive for B. anthracis by polymerase chain reaction (PCR) and a pleural biopsy was positive for B. anthracis by IHC staining. On October 13, a 54-year-old Delaware resident who worked as a mail sorter at a New Jersey postal processing and distributing center developed a painless lesion on the dorsum of his left hand. The lesion began as an erythematous "knot" several millimeters in size that developed a crusted scale during the next few days. No associated edema, eschar, or lymphadenopathy was observed. The patient had elevated levels of serum antibody (IgG) to the protective antigen component of the anthrax toxin using enzyme-linked immunosorbant assay. On October 15, a 43-year-old female postal worker who worked at a facility in which anthrax cases have been documented developed fever, headache, chills, and shortness of breath. She was treated with levofloxacin, but her symptoms progressed and she was admitted to a hospital on October 18. A chest radiograph indicated a right perihilar infiltrate and a small pleural effusion. She was started on multidrug therapy, including ciprofloxacin, which was changed to azithromycin after 24 hours. On admission, she was febrile and tachycardic. She had an elevated white blood cell (WBC) count of 11,000 with 14% bands. A CT scan on October 19 showed a right pleural effusion, perihilar consolidation, and mediastinal adenopathy. She subsequently had two thoracenteses that produced serosanguinous pleural fluid and a bronchoscopy that showed grossly edematous bronchi. Both pleural fluid and bronchial biopsy were positive for B. anthracis by IHC stain. On October 17, a 51-year-old woman developed a large pimple on her forehead with erythema and swelling. On October 18, the lesion enlarged, was slightly painful, nonpruritic, and drained a small amount of yellowish fluid. She sought medical care, cervical and preauricular lymphadenopathy was noted on physical examination, and she was treated with ciprofloxacin. The lesion progressed and ulcerated. On October 22, she presented to an emergency department and was admitted with a diagnosis of cellulitis. On admission, she was afebrile with normal vital signs and had a swollen right face and eyelid and enlarged right anterior cervical nodes. Intravenous ciprofloxacin for cutaneous anthrax was started. On October 24, the ulcer was biopsied and debrided. Biopsy specimens were positive for B. anthracis by PCR and IHC. The patient improved and was discharged on October 27 on oral ciprofloxacin. The patient worked as a bookkeeper and reported receiving no unusual or powder-containing mail at home or work. She had made no visits to any post offices in several months. District of ColumbiaTo date, investigations in the District of Columbia, Maryland, and Virginia have confirmed inhalational anthrax in four persons who worked at one postal facility in the District of Columbia. An additional case of inhalational anthrax has been confirmed in a 59-year-old postal worker in a U.S. State Department mail sorting facility that receives mail from the District of Columbia postal facility associated with the previous four cases. The patient presented to an emergency department on October 24 with temperature of 100.8 F (38 C), sweats, myalgia, chest discomfort, mild cough, nausea, vomiting, diarrhea, and abdominal pain. A chest radiograph initially was interpreted as normal but on further review indicated mediastinal widening. A CT scan showed mediastinal lymphadenopathy, hemorrhagic mediastinitis, small bilateral pleural effusions, and a small pericardial effusion. Blood cultures grew B. anthracis. The patient is receiving ciprofloxacin, rifampin, and penicillin. FloridaTo date, the investigation in Florida has identified two confirmed inhalational cases. No new cases have been identified since the last report (1). Clinical Presentation of Inhalational and Cutaneous CasesInhalational anthrax To date, CDC has identified 10 patients with confirmed or suspected inhalational anthrax associated with bioterrorism. All but the most recent patients were postal workers (six), mail handlers or sorters (two), or a journalist who were known to or believed to have processed, handled, or received letters containing B. anthracis spores. The hospital employee with inhalational anthrax did not process mail but might have carried mail to other parts of the facility. Preliminary environmental testing of the patient's work area and home was negative for B. anthracis. The investigation is ongoing. The median age of the 10 patients with inhalational anthrax was 56 years (range: 43--73 years); seven were men. The incubation period from the time of exposure to onset of symptoms when known (seven) was 7 days (range: 5--11 days). The initial illness in these patients was characterized by fever (nine) and/or sweats/chills (six) (Figure 2). Severe fatigue or malaise was present in eight and minimal or nonproductive cough in nine, including one with blood-tinged sputum. Eight patients reported chest discomfort or pleuritic pain. Abdominal pain or nausea or vomiting occurred in five, and five reported chest heaviness. Other symptoms included shortness of breath (seven), headache (five), myalgias (four), and sore throat (two). On initial presentation, total WBC count was normal or slightly elevated (7.5--13.3 x 103/cumm); however, elevation in the percentage of neutrophils or band forms was frequently noted. None of the patients had a low WBC count or lymphocytosis when initially evaluated. Chest radiograph was abnormal in all patients, but in two an initial reading was interpreted as within normal limits. Mediastinal changes including mediastinal widening, paratracheal fullness, hilar fullness, and mediastinal lymphadenopathy were noted in all eight patients who had CT scans. Mediastinal widening may be subtle, and careful review of the chest radiograph by a radiologist may be necessary. Pleural effusions were present in seven patients and were a feature of the two patients who did not have mediastinal changes on chest radiograph or did not have a CT scan. Pleural effusions often were large and hemorrhagic, reaccumulated, and required repeated thoracentesis or chest tubes. Pulmonary infiltrates were observed in four patients and were multilobar in three. Blood cultures grew B. anthracis in seven patients and in all who had not received antimicrobials. Diagnosis in the patients with negative cultures was confirmed by bronchial or pleural biopsy and specific IHC staining, by PCR of material from a sterile site, or by a fourfold rise in IgG to the protective antigen. To date, six of 10 patients with inhalational anthrax have survived. Among those whose condition was recognized early, all remain alive and two have been discharged from the hospital. Prompt recognition of the early features of inhalational anthrax is important in settings of known or suspected exposure. Cutaneous anthraxEleven patients with cutaneous anthrax have been identified in the current outbreak. Patients with cutaneous anthrax were mail handlers or sorters (four), employees of or visitors to media companies (six), and one bookkeeper. The mean incubation period for cutaneous anthrax was 5 days (range: 1--10 days) based on estimates from the postmark of letters and assumptions of dates of exposures with known positive letters or suspect letters (Figure 3). Lesions occurred on the forearm, neck, chest, and fingers (two). Lesions were painless but accompanied by a tingling sensation or pruritis. Diagnosis was established by biopsy or culture. Reported by: J Malecki, MD, Palm Beach County Health Dept, Palm Beach; S Wiersma, MD, State Epidemiologist, Florida Dept of Health. T Cahill, MD, M Grossman, MD, Columbia Presbyterian Medical Center; H Hochman, MD, M Tapper, MD, Lenox Hill Hospital; M Pomeranz, MD, A Friedman-Kien, MD, Bellevue Hospital Center; A Gurtman, MD, Mount Sinai School of Medicine, New York, New York; New York City Dept of Health. E Bresnitz, MD, State Epidemiologist, G DiFerdinando, MD, New Jersey Dept of Health and Senior Svcs. P Lurie, MD, K Nalluswami, MD, Pennsylvania Dept of Health. D Frank, MD, Greater SE Hospital; L Siegel, MD, S Adams, I Walks, MD, J Davies-Coles, PhD, District of Columbia Dept of Health. C Chiriboga, MD, Southern MD Hospital, Clinton; R Brechner, State Epidemiologist, Maryland Dept of Health and Hygiene. E Peterson, MD, Virginia Dept of Health; S Bresoff-Matcha, MD, Mid-Atlantic Permanente Medical Group and Inova Fairfax Hospital, Falls Church; M Galbraith, MD, Winchester, Virginia. J Eisold, MD, G Martin, MD, Office of the Attending Physician, US Capital. US Dept of Defense. EIS officers, CDC. Editorial Note:Since the last report (1), six new anthrax cases have been reported. Three of these cases have occupational exposures similar to previously reported cases (1). A fourth case occurred in a mail handler at a facility not previously linked to cases but that receives mail from a facility at which cases have occurred previously. Two new cases have no discernable epidemiologic link with anthrax cases previously reported or sites that are associated with known cases. These new cases suggest that anthrax exposure has occurred or is continuing to occur through means that cannot be ascribed to known contaminated letters or the paths these letters took through the mail service. The public health response to these new anthrax cases will evolve based on ongoing epidemiologic and criminal investigations. Because exposures are being intentionally perpetrated, public health authorities must be vigilant for the appearance of new cases in previously unaffected populations. Prompt data sharing between law enforcement and public health authorities is essential. Since September 11, 2001, state and local health departments have been responding to many reports of potential bioterrorist threats including letters containing powder, suspicious packages, and potential dispersal devices. During September 11--October 17, 40 state and territorial health officials who responded to a CDC telephone survey estimated that 7,000 reports had been received at their health departments, approximately 4,800 required phone follow-up, and 1,050 reports led to testing of suspicious materials at a public health laboratory (CDC, unpublished data, 2001). In comparison, the number of anthrax threats reported to federal authorities during 1996--2000 did not exceed 180 reported threats per year (Federal Bureau of Investigation, unpublished data, 2001). Therefore, although only four areas have identified cases of bioterrorism-associated anthrax, health departments throughout the nation are responding to public concerns, bioterrorism hoaxes, and threats. CDC is working with state and local health departments and the U.S. Postal Service to develop standardized guidelines for identifying populations that should receive antimicrobial prophylaxis for prevention of inhalational anthrax. Current challenges include identifying factors that promote the aeresolization of B. anthracis in mail-handling facilities and assessing the risk for anthrax in environments contaminated with B. anthracis spores. Safe levels of B. anthracis spore contamination in occupational settings must be defined to determine the need for clean-up of contaminated facilities. The current antimicrobial prophylaxis recommendations address the prevention of inhalational anthrax, but CDC also is evaluating measures to prevent cutaneous anthrax. Postexposure prophylaxis with a recommended antimicrobial agent for the prescribed period of time can prevent inhalational anthrax. In the case of a known contaminated letter sent to the office of a U.S. Senator, antimicrobial prophylaxis was administered to persons from the area of exposure and first-responders to the incident (1). To date, there have been no cases of anthrax, even among those who had the greatest exposure. Antimicrobial prophylaxis had been recommended for the U.S. State Department mail handler with anthrax, but the worker had not started treatment before the onset of illness. Public health response must include prompt initiation of prophylaxis for exposed persons and systems to promote adherence to a full 60-day regimen. Previous guidelines recommended ciprofloxacin for antimicrobial prophylaxis until antimicrobial susceptibility test data was available (3). Isolates involved in the current bioterrorism attacks have been susceptible to ciprofloxacin, doxycycline, and several other antimicrobial agents. Considerations for choosing an antimicrobial agent include effectiveness, resistance, side effects, and cost. No evidence demonstrates that ciprofloxacin is more or less effective than doxycycline for antimicrobial prophylaxis to B. anthracis. Widespread use of any antimicrobial will promote resistance. Many common pathogens are already resistant to tetracyclines such as doxycycline. However, fluoroquinolone resistance is not yet common in these same organisms. To preserve the effectiveness of fluoroquinolone against other infections, use of doxycycline for prevention of B. anthracis infection among populations at risk may be preferable. However, the selection of the antimicrobial agent for an individual patient should be based on side-effect profiles, history of reactions, and the clinical setting. CDC and state and local public health agencies continue to mobilize epidemiologic, laboratory, and other staff to identify and investigate acts of bioterrorism. Cases of bioterrorism-associated anthrax continue to occur and new risk populations may be identified. Until the cause of these acts are removed, public health authorities and clinicians should remain alert for cases of anthrax. References
Figure 1 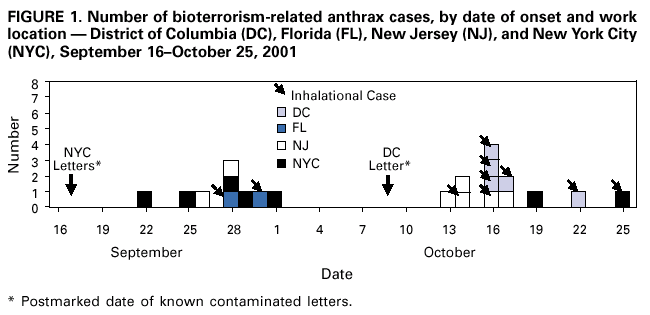 Return to top. Figure 2 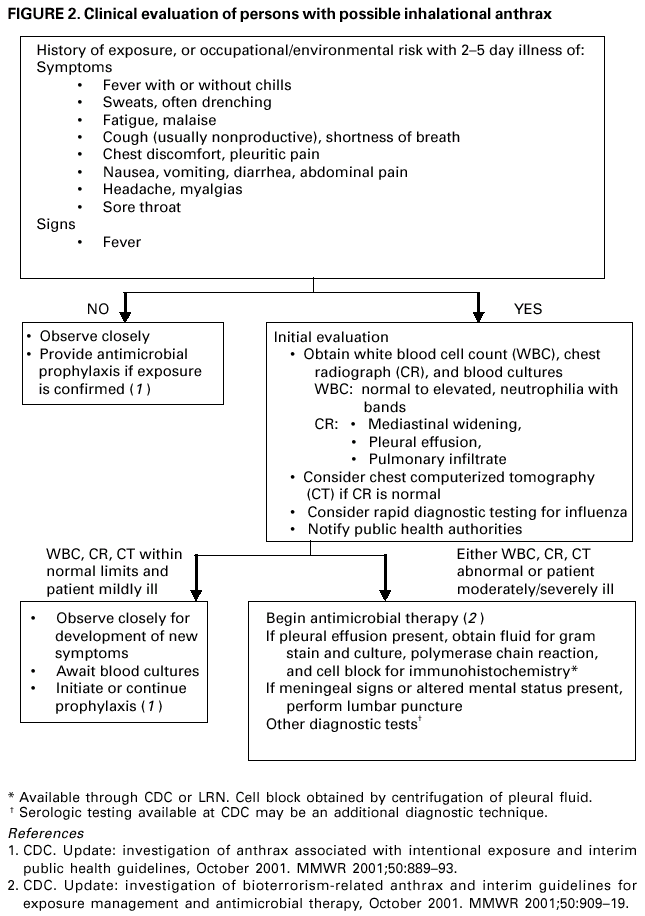 Return to top. Figure 3 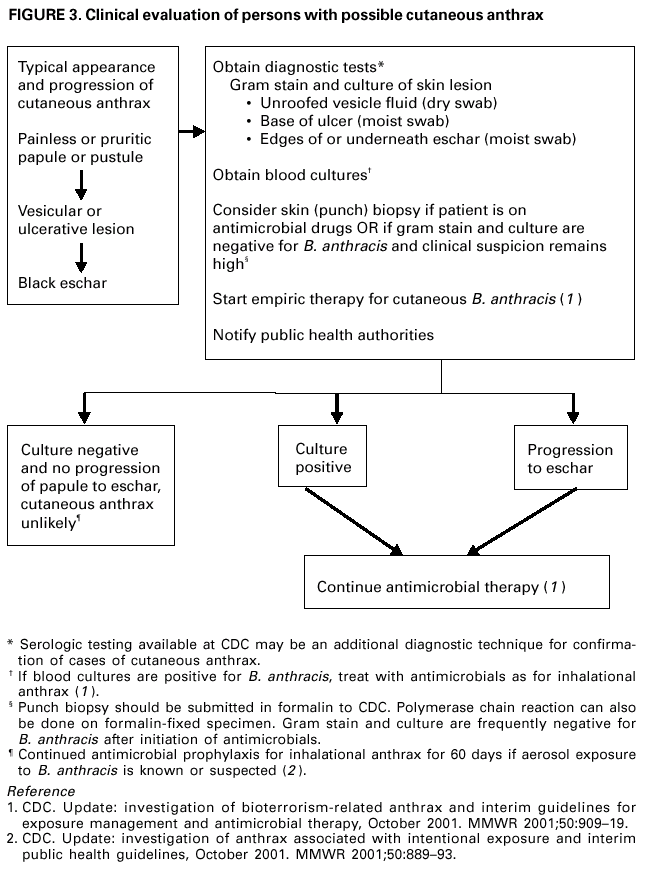 Return to top.
Disclaimer All MMWR HTML versions of articles are electronic conversions from ASCII text into HTML. This conversion may have resulted in character translation or format errors in the HTML version. Users should not rely on this HTML document, but are referred to the electronic PDF version and/or the original MMWR paper copy for the official text, figures, and tables. An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S. Government Printing Office (GPO), Washington, DC 20402-9371; telephone: (202) 512-1800. Contact GPO for current prices. **Questions or messages regarding errors in formatting should be addressed to mmwrq@cdc.gov.Page converted: 11/2/2001 |
|||||||||
This page last reviewed 11/2/2001
|