 |
|
|
|
|
|
|
| ||||||||||
|
|
|
|
|
|
|
||||
| ||||||||||
|
|
|
|
|
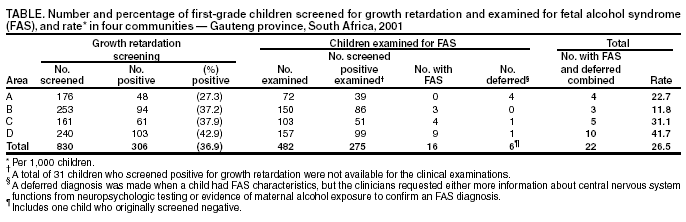
Persons using assistive technology might not be able to fully access information in this file. For assistance, please send e-mail to: mmwrq@cdc.gov. Type 508 Accommodation and the title of the report in the subject line of e-mail. Fetal Alcohol Syndrome --- South Africa, 2001Fetal alcohol syndrome (FAS) is caused by maternal alcohol use during pregnancy and is one of the leading causes of preventable birth defects and developmental disabilities (1). The FAS phenotype is characterized by a combination of facial dysmorphic features, growth retardation, and central nervous system (CNS) abnormalities. State-based estimates of the prevalence of FAS in the United States vary from 0.3 to 1.5 per 1,000 live-born infants (2). Recently, the highest prevalence of FAS worldwide was reported among first-grade children in a wine-growing region in the Western Cape province of South Africa (3). Investigators for the National Institutes of Alcoholism and Alcohol Abuse (NIAAA) reported a FAS prevalence of 40.5 to 46.4 per 1,000 children aged 5--9 years in one community in Western Cape. To determine whether FAS was associated exclusively with the wine-growing region in Western Cape or was more endemic in other areas of the country, CDC, in collaboration with the University of Witwatersrand and the Foundation for Alcohol Related Research in Johannesburg, South Africa, conducted a prevalence study in Gauteng province and is developing ongoing surveillance and prevention activities. This report summarizes the findings of the study, which indicate a high prevalence of FAS among first-grade children in four nonwine-growing communities around Johannesburg. Because South Africa has limited resources and many competing health problems (e.g., human immunodeficiency virus/acquired immunodeficiency syndrome, tuberculosis, and sexually transmitted diseases), integrating prenatal alcohol-exposure prevention activities with existing prevention programs should be explored. Four communities were selected on the basis of their willingness to participate and ability to represent the various racial/ethnic groups in Gauteng province. Human subjects approval was obtained through the University of Witwatersrand's Institutional Review Board. A two-stage screening and case-finding method, similar to methods implemented in the Western Cape communities, was used to identify children with FAS (3). Because growth retardation is a cardinal feature of FAS, in the first stage (screening), the weights, heights, and head circumferences of all children in first grade in selected schools were measured. Because standard growth curves are not available for children in South Africa, the World Health Organization (WHO) international growth reference curves were used for weight and height (4,5). Head circumference growth reference curves developed from the Fels Longitudinal Study were used to determine 10th-percentile cut points by age and sex (6). Children were classified as screen-positive if they were below the 10th percentile for weight and height or head circumference for age and sex. The sensitivity of the screening procedures in this study was 96%; however, the positive predictive value was only 8%. In the second stage (case ascertainment), all screen-positive children and, whenever possible, screen-negative children who were matched by age, sex, and classroom received a physical evaluation by two teams of physicians. The physicians were trained in clinical genetics and FAS diagnosis and were unaware of the screening team's findings during the clinical evaluation of the children. Physical signs of FAS (e.g., facial dysmorphia, joint and bone abnormalities, CNS anomalies, and skin and other abnormalities) and other measurements (e.g., palpebral fissures and inner canthal and interpupillary distances) were recorded, and a diagnosis of "FAS," "deferred," or "not FAS" was determined. The physicians diagnosed FAS on the basis of clinical judgment and the presence of abnormalities in three major case-definition categories: growth retardation (i.e., <10th percentile for weight and height); facial dysmorphic features (e.g., hypoplastic midface, smooth philtrum, thin narrow upper lip, flat nasal bridge, and small palpebral fissures); and head circumference below the 10th percentile (1). A deferred diagnosis was made when a child had FAS characteristics, but the clinicians requested either more information about CNS functions from neuropsychologic testing or evidence of maternal alcohol exposure to confirm an FAS diagnosis. A "not FAS" diagnosis was indicated when a child did not appear to have the phenotype associated with prenatal alcohol exposure. All children with an FAS or deferred diagnosis received a follow-up neuropsychologic assessment to measure CNS-related disabilities and cognitive functioning to make appropriate referrals and recommendations for services. After completion of all examinations, clinicians held a case conference for each child to determine a final diagnosis. Six children with physical signs of FAS remained in the deferred case category pending further neuropsychologic assessments. Among 19 participating schools from the four communities, 830 children in first grade were screened for growth retardation (Table). The median age of children screened was 6.5 years (range: 5--10 years). A total of 306 (37%) children screened positive for weight and height or for head circumference below the 10th percentile. The percentage of screen-positive children varied among the four communities from 27% to 43%. Of the 306 screen-positive children, 275 (90%) were available for the FAS clinical evaluations. For purposes of comparison, another 207 children from the screen-negative group were included; for three schools, >50% of children screened positive, limiting the pool of screen-negative children available for comparison. A total of 482 children received clinical evaluations. Of the 275 screen-positive children examined, 21 (8%) received an FAS (n = 16) or deferred (n = five) diagnosis. A deferred diagnosis was made on one child from the screen-negative group; however, none from the screen-negative group had FAS diagnosed. The median prevalence for FAS alone among first-grade children in the four communities was 19 per 1,000 children (range: 0--37.5). When FAS and deferred diagnoses were combined, the median prevalence was 26.5 per 1,000 children (range: 11.8--41.7) in the four communities (Table). Reported by: D Viljoen, MD, P Craig, Univ of Witwatersrand and the Foundation for Alcohol Related Research, Johannesburg, South Africa. K Hymbaugh, MPH, C Boyle, PhD, National Center on Birth Defects and Developmental Disabilities; S Blount, MD, Office of Global Health, CDC. Editorial Note:The findings of this report are comparable to an earlier report of high prevalence of FAS in the Western Cape province (3) and confirm that FAS is a serious public health problem in South Africa. This report extends the initial findings (3), indicating that the unusually high prevalence of FAS among first-grade children is not a function of the availability of alcohol in the wine-growing regions of South Africa. Although rates of FAS in all four communities described in this report were high, the rates varied by community and probably reflect differences in local drinking patterns, alcohol availability, poverty, unemployment, health problems, and other risk factors. In the absence of national standard anthropometric references for growth, WHO recommends the use of growth curves by using data from the Fels Research Institute and U.S. Health Examination Surveys for international studies (4,7). In the study described in this report, which used these growth references, a high proportion of children screened positive either for weight and height or for head circumference below the 10th percentile for sex and age. Growth retardation is a cardinal feature of the FAS phenotype and appears to be an important tool for screening in this population. Although the sensitivity of the screening procedures was high (96%), the positive predictive value was only 8%, indicating a large number of children without FAS were examined. Because measuring weight and height is relatively easy, can be performed by local staff, and can be cost-effective by eliminating unnecessary clinical examinations of children who do not meet the growth retardation criteria for FAS, using growth measures for FAS screening in a high-prevalence population is advantageous. However, staff time and clinical examinations are completed on many children who do not have FAS. The population-based growth data collected on all first graders in the four communities can be used to refine the screening tool and improve the positive predictive value by adjusting the screen-positive percentile cut points for future FAS screening, case-finding, and surveillance activities in South Africa. The findings in this report are subject to at least two limitations. First, the number of children in South Africa affected adversely by in-utero alcohol exposure is probably underestimated. Many children with severe FAS might not attend public schools, or, because of the increased health problems associated with this birth defect, might have died before school-entry age. In addition, the programs for children with developmental disabilities that exist in the study areas were not used for case-finding. Second, because children were not identified until school entry, they missed opportunities for early education interventions that could improve overall developmental outcome. The late age at identification represents missed opportunities to intervene with high-risk mothers who might have given birth to additional children with FAS. Ideally, case-finding should be conducted at birth to maximize the opportunity for prevention and early intervention with the family. With limited resources and many health problems in South Africa, prenatal alcohol-exposure prevention activities for women and intervention programs for children with FAS are virtually nonexistent. The screening and case-finding approach described in this report can be useful for identifying high-risk communities and targeting scarce prevention resources. As resources become available for prevention and intervention activities, an ongoing cost-effective surveillance system that maximizes case-finding in a child's first year of life is crucial to evaluate prevention strategies and programs targeted toward South Africa's multiple racial/ethnic groups. CDC will work with investigators in South Africa to improve the screening criteria, evaluate strategies to identify children earlier in life, and develop and evaluate prevention and intervention strategies. References
 Return to top.
Disclaimer All MMWR HTML versions of articles are electronic conversions from ASCII text into HTML. This conversion may have resulted in character translation or format errors in the HTML version. Users should not rely on this HTML document, but are referred to the electronic PDF version and/or the original MMWR paper copy for the official text, figures, and tables. An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S. Government Printing Office (GPO), Washington, DC 20402-9371; telephone: (202) 512-1800. Contact GPO for current prices. **Questions or messages regarding errors in formatting should be addressed to mmwrq@cdc.gov.Page converted: 7/17/2003 |
|||||||||
This page last reviewed 7/17/2003
|