 |
|
|
|
|
|
|
| ||||||||||
|
|
|
|
|
|
|
||||
| ||||||||||
|
|
|
|
|
Persons using assistive technology might not be able to fully access information in this file. For assistance, please send e-mail to: mmwrq@cdc.gov. Type 508 Accommodation and the title of the report in the subject line of e-mail. Routinely Recommended HIV Testing at an Urban Urgent-Care Clinic --- Atlanta, Georgia, 2000In 1993, CDC recommended that hospitals and associated clinics in areas with high human immunodeficiency virus (HIV) prevalence offer HIV testing routinely to all patients aged 15--54 years (1). Although voluntary routine screening among hospitalized (2) and emergency department patients (3) can identify many undiagnosed HIV-infected persons, few screening programs have been implemented in these settings. A 1997 study at Grady Memorial Hospital, Atlanta, Georgia, found that nearly two thirds of inpatients newly diagnosed with acquired immunodeficiency syndrome (AIDS) had received medical care within the Grady health system during the 12 months preceding admission* (4); these previous encounters were missed opportunities for earlier diagnosis of HIV. In response to the 1997 study, investigators studied routinely recommending HIV testing to patients presenting to the urgent-care clinic, an ambulatory clinic that provides episodic medical care to indigent and low income adults. This report summarizes the results of that study in which, compared with 1999 when testing was based on symptoms or risk behaviors, more patients were tested for HIV, more HIV infections were detected, and more infected persons learned their diagnosis and entered into care. These results reflect the benefits of recommending HIV testing routinely to patients in medical facilities located in areas with high HIV prevalence. For 24 weeks (i.e., March 20--September 1, 2000), clinicians were encouraged to recommend HIV testing to all urgent-care clinic patients aged 18--65 years who were neither known to be HIV seropositive† nor tested during the preceding 6 months. These 24 weeks were compared with testing during the same 24 weeks in 1999, when HIV testing was conducted only when clinicians were concerned about patients' symptoms or risk behaviors. During the study period, posters encouraging patients to be tested for HIV were displayed prominently, and patients received a brochure about HIV and HIV testing before discussions with their heath-care providers. Patients who accepted testing provided written consent and were not charged for HIV testing, which was conducted with either a rapid test (Single Use Diagnostic System [SUDS] HIV-1 Test [Abbott-Murex Corporation, Norcross, Georgia]) or a standard enzyme immunoassay (EIA). All SUDS tests were supplemented with EIA; all positive SUDS and EIA tests were confirmed with Western blot. Clinicians, counselors, or study investigators trained in HIV counseling delivered test results; a physician's assistant telephoned or wrote to HIV-seropositive persons who had left before their SUDS results were available or who did not return to the clinic for their EIA result. The study was approved by the human subjects research committees of CDC, Emory University, and the Grady Research Oversight Committee. Patients were defined as knowing their test result if discussion of results was documented in the medical record or clinic HIV testing log or if patients had a CD4 test within 2 months after their positive HIV test. Entry into care was defined by a record of a visit to the Grady infectious disease clinic within 4 months following the positive HIV test. Approximately 20,000 clinic visits occurred during each of the two periods (i.e., 1999 and 2000) (Table 1). Comparing 2000 with 1999, 1687 more patients were tested, 27 more infections were newly detected, 27 more patients were informed of their HIV-positive test result, and twice as many HIV-seropositive patients (26 versus 13) entered into care§ (Table 1). During the study, infected persons may have had HIV detected at an earlier stage of infection; 28 (67%) of 42 persons had a CD4+ T cell count >200 cells/µL during the study period compared with 10 (45%) of 22 during 1999 (p=0.1). Additional information on HIV test eligibility, provider recommendations, and testing patterns was collected from 8 a.m. to 5 p.m. weekdays during the study period¶. Among the 13,039 patient visits to the urgent-care clinic during these hours, 10,719 were eligible to be offered HIV testing. Among those eligible, 6421 (60%) were offered testing and 2564 (40%) accepted. Among those who accepted testing, 1839 (72%) were actually tested. Among 886 patients tested with SUDS, 236 (27%) received results the same day. Reported by: C Del Rio, MD, C Franco Paredes, MD, W Duffus, MD, K Cesarz, S Green, G Hicks, MPH, M Barragan, MPH, Grady Memorial Hospital, Atlanta, Georgia. Div of HIV/AIDS Prevention, National Center for HIV, STD, and TB Prevention; and an EIS Officer, CDC. Editorial Note:HIV testing usually relies on a patient's request or a health-care provider's concern about symptoms or risk behaviors. This report indicates that when providers at an urgent-care clinic in a high prevalence area routinely recommended HIV testing, more persons were tested, more HIV infections were detected, and more patients with newly detected infections learned their diagnosis and entered into care. Patients often were diagnosed earlier in the course of their infection. Despite the benefits of routinely recommended testing, barriers to this approach exist, as demonstrated by the proportion of patients who were not offered testing, did not accept testing, and were not tested once they had accepted. In addition, 26% of patients with newly detected infections did not learn their HIV-positive diagnosis, and 53% of those who learned their diagnosis did not enter into medical care. The findings in this report are subject to at least four limitations. First, some newly diagnosed patients may have sought care from providers outside the Grady health system (e.g., private providers or other public health facilities) and would not have been recorded as having received care. Second, the large proportion of patients tested during both periods for whom CD4 count data were unavailable limited the comparison of the stage of infection among patients diagnosed in 1999 with those diagnosed in 2000. Third, the proportions of patients who were eligible for, offered, accepted, and were actually tested from 8 a.m. to 5 p.m. weekdays may have differed from the 1999 comparison period or other study hours. Finally, no data were available to evaluate whether characteristics of the clinic population changed between comparison periods. The findings in this study suggest some strategies clinics can use to increase the acceptance, feasibility, and effectiveness of routinely recommended testing. To increase the numbers of patients providers recommend for testing, providers must be convinced that time demands will not be excessive; to increase the number of patients who accept testing, patients must believe that HIV testing and the subsequent results are relevant. HIV risk can be assessed quickly using screening questions, and patients can be referred for client-centered prevention counseling when necessary (5). In this study, posters and brochures provided basic HIV test information and helped providers focus on issues specific to the individual patient. Rapid tests that could be performed in the clinic rather than a hospital laboratory and that could use either oral fluids or whole blood obtained by fingerstick** might increase the acceptability of HIV testing and the number of patients that receive test results in a clinic. In addition, medical centers must develop clear, concise strategies that would facilitate medical care and prevention counseling for newly diagnosed patients. Convenient and efficient links to HIV medical care are benefits to having HIV testing in a clinic; however, informing patients of their diagnosis is insufficient to ensure that they will receive HIV-specific medical care. Testing for HIV infection in high HIV prevalence areas has become more important and more feasible since 1993. Medical therapy now can reduce substantially HIV-related morbidity and mortality, prevention counseling can help HIV-infected persons protect their partners by adopting safer behaviors, and earlier HIV diagnosis increases the benefits of both treatment and prevention (6). Approximately 300,000 HIV-infected persons in the United States may not know that they are infected (7), and missed opportunities for earlier diagnosis of HIV frequently occur in medical settings (4). Recommending HIV testing routinely in clinical settings presents an opportunity to target high prevalence communities, destigmatize HIV testing, and better link HIV-infected persons to care and prevention services. Counseling and testing are potentially cost saving because they can reduce transmission (8); however, institutions are unlikely to absorb these costs. Public health departments and other HIV prevention programs can assist with financial and/or human resources in implementing routinely recommended HIV testing at clinics in high HIV prevalence areas. Health departments and administrators of clinical facilities in such areas are encouraged to adopt a policy of routinely recommending HIV testing. References
*Median of four visits per patient; the most frequented departments were the emergency department and the urgent-care clinic. † Based on patient interview and medical record review. § This intervention was neither designed nor expected to improve the proportion of infected persons who entered into care; the proportion was approximately the same for the two periods (i.e., 13 [46%] of 28 in 1999 and 26 [47%] of 55 in 2000). ¶ Urgent-care clinic hours during 1999 and 2000 were Monday--Friday from 8 a.m. to 10 p.m. and weekends from 9 a.m. to 7 p.m. ** Such tests would eliminate the need to wait for a phlebotomist, have blood drawn, and return for a second visit to receive test results. SUDS, the only rapid HIV test licensed in the United States, is labor intensive, and most patients tested with SUDS in this study did not receive their SUDS result on the same day that it was performed. Table 1 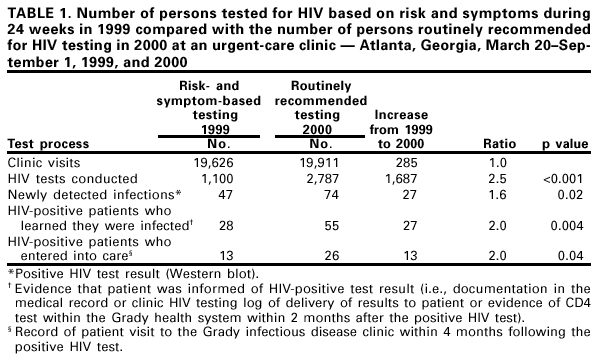 Return to top. Disclaimer All MMWR HTML versions of articles are electronic conversions from ASCII text into HTML. This conversion may have resulted in character translation or format errors in the HTML version. Users should not rely on this HTML document, but are referred to the electronic PDF version and/or the original MMWR paper copy for the official text, figures, and tables. An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S. Government Printing Office (GPO), Washington, DC 20402-9371; telephone: (202) 512-1800. Contact GPO for current prices. **Questions or messages regarding errors in formatting should be addressed to mmwrq@cdc.gov.Page converted: 7/2/2001 |
|||||||||
This page last reviewed 7/2/2001
|