Japanese Encephalitis Vaccine Evidence to Recommendations
Printer friendly version of this page [1 MB, 46 pages]
Policy question
The primary policy question was “Should inactivated Vero cell culture-derived Japanese encephalitis (JE) vaccine (JE-VC) be recommended for use in persons aged ≥2 months at risk of travel-related exposure to JE virus?”
- Population: Persons aged ≥2 months traveling to JE risk areas
- Intervention: JE-VC administered as a 2-dose primary series
- Comparison: No JE vaccination
- Outcomes: The benefits considered critical outcomes for which there were data available included short and long-term seroprotection using the established immunologic correlate of protection (JE virus neutralizing antibodies at a PRNT50 titer ≥10) (Table 1). The harms considered critical outcomes were serious adverse events and adverse events of special interest (i.e., fever, rash, hypersensitivity/urticaria, neurologic adverse events, and medically attended adverse events).
Background
JE is a mosquito-borne disease that occurs throughout most of Asia and parts of the western Pacific. JE virus is transmitted in an enzootic cycle between mosquitoes and amplifying vertebrate hosts, primarily pigs and wading birds. JE virus is transmitted to humans by infected mosquitoes. Humans usually do not develop a level or duration of viremia sufficient to infect mosquitoes, and therefore are considered dead-end hosts. JE virus transmission occurs primarily in rural agricultural areas.
JE-VC (manufactured as IXIARO) is the only JE vaccine licensed and available in the United States. JE-VC is manufactured by Valneva Austria GmbH. In March 2009, the US Food and Drug Administration (FDA) licensed JE-VC for use in adults aged ≥17 years. In June 2009, the Advisory Committee on Immunization Practices (ACIP) approved recommendations for use of JE-VC in adults [Fischer 2010]. In September 2010, FDA approved a JE-VC booster dose for adults and, in February 2011, adult booster dose recommendations were approved [CDC 2011]. In May 2013, FDA approval for use of JE-VC was extended to include children aged 2 months through 16 years. A Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) for use of JE-VC in children was presented to ACIP and recommendations for pediatric use of JE-VC were approved in June 2013. In April 2018, FDA approval for a booster dose was extended to include the pediatric age group.
There are no efficacy data for JE-VC. However, a JE virus 50% plaque reduction neutralization test (PRNT50) titer of ≥10 is an established immunologic correlate of protection [Markoff 2000; Hombach 2005]. JE-VC was licensed based on its ability to induce neutralizing antibodies and a non-inferiority comparison to a licensed inactivated mouse brain-derived JE vaccine (JE-MB [manufactured as JE-VAX]). JE-MB is no longer available in the United States. At the time of licensure, JE-VC had been studied in <5,000 adults. Since JE-VC’s licensure, more than 1 million doses have been distributed in the United States. Since the 2013 GRADE, additional immunogenicity and safety data from clinical trials and surveillance activities have become available. The ACIP JE Vaccine Work Group used GRADE methods to review and evaluate these newly available data [Ahmed 2013]. Additional factors also were assessed in considering JE vaccine recommendations, as outlined in the Evidence to Recommendations (EtR) framework [Lee 2018]. The results of the work group’s deliberations and the proposed JE vaccine recommendations are presented below.
Domains
Domain 1. The Problem
Criterion: Public Health Priority
Criterion question: Is the problem of public health importance?
JE virus is the leading vaccine-preventable cause of encephalitis in Asia, with an estimated 67,900 cases annually [Campbell 2011]. In the highest risk areas in Asia prior to vaccination programs, incidence rates as high as 20 cases per 100,000 children per year were reported. Risk areas for JE virus transmission include most of Asia and parts of the Western Pacific. JE clearly is of public health importance for residents in these regions.
While JE incidence is high in some JE-endemic regions, JE cases are infrequently reported in the United States. A JE vaccine was first licensed in the United States at the end of 1992. In the 25-year period from 1993–2017, 12 JE cases were reported among U.S. tourists or expatriates. All cases occurred in 2003 or later and none were immunized. There was a median of 0 cases reported per year (range: 0–2 cases). Based on the 12 reported cases from 1993‒2017 and approximately 4‒5 million U.S. citizen trips annually to Asia during that time period, the overall estimated JE risk for a U.S. traveler is <1 case per million trips to Asia. Therefore, JE cannot be considered a substantial public health problem for U.S. travelers overall.
JE virus is not found in the United States. When humans are infected with JE virus, they have a low level viremia and so are considered “dead end” hosts. Therefore, the risk is extremely low for subsequent local transmission of JE virus in the United States from an infected traveler, and importation of JE virus by an infected traveler is not a public health concern.
While the risk for JE in most travelers is low, it varies based on travel duration, season, location, activities, and accommodations. Vaccination will be of benefit to some travelers. For some persons, such as those taking up long term residence in rural areas of Asia, risk might approach a similar level to populations of endemic areas. There are examples of substantial JE disease risk among certain groups. For example, more than 300 cases of JE were reported among soldiers deployed to Asia from the United States, the United Kingdom, Australia, and Russia in the period before JE vaccine was widely available and utilized among military personnel.
Most JE virus infections in humans are asymptomatic; <1% of infected people develop encephalitis. However, when disease occurs, outcome is often severe, with a 20–30% mortality rate, and 30–50% of survivors having significant neurologic, cognitive, or behavioral sequelae. There is no antiviral therapy and treatment consists of supportive care. Given its substantial morbidity and mortality, JE can be considered a serious problem for individual travelers. Substantial resources might be needed to care for a person with a serious long-term disability.
Finally, JE vaccine is paid for out-of-pocket by most U.S. civilian travelers. It is typically not covered by private insurers and is not covered under the Vaccines for Children program. Therefore, the costs and benefits of immunization are primarily at the individual rather than societal level.
Conclusion
The work group agreed JE is a public health problem in JE endemic countries. For the U.S. population, it is an individual traveler rather than societal concern, so the question of public health importance is not directly applicable. In addition, JE vaccine is typically paid for out-of-pocket, unlike vaccines in the national immunization program. However, since certain travelers might have a sufficiently high risk to warrant vaccination, the consensus was that the public health importance “varies” related to the individual person’s itinerary and activities.
Domain 2. Benefits and Harms
Methodology for GRADE
GRADE was used to assess the benefits and harms of JE-VC. To identify published literature that contained relevant evidence, we conducted a search of Medline, Embase, CINAHL, and Cochrane Library databases for papers in any language published from May 2013 through February 28 2018. The date limits were selected to provide an update to the literature search conducted for the previous GRADE for use of JE-VC in children, which covered the period January 2006 through April 2013. We used the following search strategy and keywords: “Japanese encephalitis” and “vaccine” and “IXIARO or JESPECT or IC51 or JEEV or Vero or purified inactivated”. The title and abstract of the studies were reviewed to identify relevant articles; if no abstract was provided, the paper itself was reviewed.
Articles that presented data on JE-VC were included if they met the following criteria: 1) Involved human subjects; 2) Reported primary data; 3) Included data relevant to the outcome measures being assessed (i.e., vaccine efficacy, seroprotection at 1 and 6 months after vaccination, serious adverse events, or adverse events of special interest); and 4) Included data for an FDA-approved dose (i.e., 0.25mL for children aged 2 months through 2 years and 0.5mL for children and adults aged ≥3 years). Publications that met the above criteria but represented a single case report were excluded.
We identified 479 records using the search strategy. Among these studies, 466 were excluded as they did not include any JE-VC data or did not present primary data. Four studies that included JE-VC human data also were excluded: 1) Two studies that presented safety or immunogenicity data at ≥7 months post-vaccination (i.e., outside the timeframe of interest); 2) One study that only presented data on cross-protective capacity of JE-VC against various JE virus genotypes among a cohort of travelers described in a separate publication; and 3) One study that included study groups that received JE-VC and rabies vaccine with or without meningococcal vaccine but did not include a study group that received JE-VC alone. Following the 470 exclusions, nine studies remained; these were combined with the 12 studies identified in the evidence retrieval process for the previous GRADE assessment [ACIP 2013]. In total, data from 21 published studies were included in this GRADE evaluation.
Unpublished data also were considered for JE-VC and another similar inactivated Vero cell culture-derived JE vaccine (JEEV) manufactured by Biological E (Hyderabad, India). JEEV is manufactured with technology licensed from Valneva. JEEV and IXIARO use the same virus strain, adjuvant, and virus purification; however, no process comparability studies have been completed and it cannot be assumed that the two final vaccine products are the same. JEEV is approved in India for use in children aged 1 through 2 years (two 0.25mL doses administered 28 days apart) and adults aged 18 through 49 (two 0.5mL doses administered 28 days apart) [Central Drugs Standard Control Organization 2013]
Evidence type was assessed through a review of study design, risk of bias, inconsistency, indirectness, imprecision, and other considerations (i.e., publication bias, strength of association, dose response, or opposing plausible residual confounding).
Criterion 1: Magnitude of desirable anticipated effects
Criterion question: How substantial are the desirable anticipated effects?
Seroprotection at 1 month
The evidence used to evaluate seroprotection at 1 month after vaccination with JE-VC was from 12 studies, including four randomized controlled trials (RCTs) (Table 2). Of the 1,673 JE-VC recipients in the 12 studies combined, 1,582 (95%) achieved seroprotection levels at 1 month after the 2-dose primary series. The only study with a seroprotection rate <95% was conducted among a cohort of older adults aged ≥64 years in which 128 (65%) of 197 participants achieved seroprotection levels at 42 days after the 2-dose series. These data on immunogenicity in older adults were considered by the work group and presented to ACIP in October 2015 and were submitted to FDA. While there are lower seroprotection rates in older adults compared to younger adults, there are no data on safety, immunogenicity, or optimal timing of a possible third primary series dose or early booster dose for older adults.
In the four RCTs, seroprotection rates for JE-VC recipients were similar to or higher than seroprotection rates for recipients of the comparator vaccines. When data from the four RCTs were combined and weighted using a random effects model, there was no significant difference in seroprotection rates between recipients of JE-VC and the other JE vaccines (Figure 1).
In addition to the studies of JE-VC, we reviewed evidence for seroprotection in one RCT performed using a similar JE vaccine (JEEV) among children aged 1 and 2 years in India [Biological E 2013] (Table 3). The findings were similar to those seen with JE-VC.
Seroprotection at 5 to 6 months
The evidence used to evaluate seroprotection at 5 to 6 months after vaccination with JE-VC was from six studies of JE-VC, including two RCTs (Table 4). Of the 941 JE-VC recipients in the six studies combined, 864 (92%) achieved seroprotection levels at 5 to 6 months after the 2-dose primary series. Seroprotection rates in the individual studies ranged from 83% to 100%. The findings from the two RCTs in adults showed that a significantly higher proportion of JE-VC recipients achieved seroprotection levels at this time point compared with subjects who received mouse brain-derived JE vaccine (Figure 2).
Additional information
The work group noted that the majority of data are in adults; however, data from the three pediatric studies supported by adult data were considered sufficient for pediatric licensure. While herd immunity is important for some vaccines, it is not a consideration for immunization against JE, as JE virus circulates in an enzootic cycle in the environment and is not transmitted from person-to person.
Conclusion
On the basis of these seroprotection data at 1 and 5– 6 months after the primary series of JE-VC, the work group concluded the desirable anticipated effects were “large”.
Criterion 2: Magnitude of undesirable anticipated effects
Criterion question: How substantial are the undesirable anticipated effects?
Serious adverse events
The evidence used to evaluate serious adverse events following JE-VC was from 16 studies, including 12 clinical trials and four post-marketing assessments that included data from three countries. Among the 12 clinical trials, eight were RCTs and four were observational studies (Table 5). Any serious adverse events within 1 month after either JE-VC dose were reported in 29 (1%) of the 4,855 subjects in these 12 clinical trials. Serious adverse events within 6 to 7 months after the first dose were reported in 72 (1%) of 5,269 subjects included in four clinical trials (Table 6). Although the relatively small numbers of subjects in these clinical trials limit the ability to detect rare serious adverse events, post-marketing surveillance data following distribution of >1 million doses provide indirect but reassuring support for the vaccine’s safety. Three large post-marketing surveillance evaluations reported 1.1 to 1.8 serious adverse events per 100,000 doses distributed (Table 7). These reported rates are similar to or lower than rates of serious adverse events per 100,000 doses distributed from post-marketing adverse event surveillance for other vaccines, including quadrivalent human papillomavirus vaccine (1.9 per 100,000 doses), 23-valent pneumococcal polysaccharide vaccine (2.0), yellow fever vaccine (3.8), and live attenuated herpes zoster vaccine (4.4)[Arana 2018, Miller 2016, Lindsey 2016, Miller 2018]. In one small postlicensure study among children, a retrospective chart review for medical visits following administration of JE-VC to 145 children in a travel clinic detected no serious adverse events.
When data from the eight RCTs were combined and weighted using a random effects model, there was no significant difference in proportions of subjects with serious adverse events within 1 month of JE-VC or the comparison vaccines (Figure 3). The risk ratio for serious adverse events within 6 to 7 months after the first dose of JE-VC or control vaccine from two studies was 0.7, but the result was non-significant (Figure 4). No patterns in the timing or types of serious adverse events were identified in the clinical trials or surveillance data.
Adverse events of special interest
The evidence used to evaluate adverse events of special interest (i.e., fever, rash, hypersensitivity/urticaria, neurologic, and medically attended adverse events) following JE-VC was from 12 studies, including eight clinical trials and four post-marketing assessments. Fever within 7 days after either JE-VC dose was reported in 296 (8%) of 3,892 subjects in seven studies; proportions of subjects with fever in individual studies ranged from 0% to 21% (Table 8). The differences in proportions were likely related to several factors, including the different age groups studied, variable locations of study sites (i.e., Europe, United States, Australia, India, and the Philippines), differences in study methodology, and the different study population sizes with less precision in some smaller studies. In the two studies with the highest fever rates for JE-VC recipients, there were no significant differences in fever rates for recipients of the control vaccines. Rash within 7 days after either JE-VC dose was reported in 81 (2%) of 3,892 subjects, with proportions in individual studies all ≤4% (Table 9). Hypersensitivity or urticaria within 1 month of either dose was reported in 15 (<1%) of 3,868 JE-VC recipients in six studies, with proportions ≤5% in all studies (Table 10). Neurologic adverse events (excluding headache) within 1 month of either JE-VC dose were reported in 26 (1%) of 3,668 recipients, and proportions were ≤1% in each of the studies (Table 11). Medically attended adverse events within 1 month after either dose were reported in 571 (14%) of 3,947 subjects. The proportion of subjects with medically attended adverse events in individual studies ranged from 0 through 19%; the two studies with the highest percentage of subjects with medically attended adverse events were conducted among children in the Philippines and elderly adults in Europe (Table 12).
In passive post-marketing surveillance, the reported incidence of hypersensitivity was 3.0 to 4.4 per 100,000 doses distributed (Table 13) and neurologic adverse events (excluding headache) was 0.2 to 1.1 per 100,000 doses distributed (Table 14). In a post-marketing adverse event surveillance study conducted among military personnel and involving retrospective review of medical records, the rate of hypersensitivity reactions was 24.8 per 100,000 doses administered and the rate of neurologic events was 22.0 per 100,000 doses administered. These much higher rates reflected the different methodology used in the study. An active surveillance approach was used and events were identified using ICD-9 codes but complete descriptions of events often were lacking, preventing clarification of the nature of some events. In addition, the assessment was conducted among military personnel who were often given multiple other vaccines concomitantly, including some reactogenic vaccines (e.g., anthrax and smallpox vaccines).
When data from the RCTs were combined and weighted using a random effects model, there were no significant differences in the proportions with any of these adverse events of special interest between recipients of JE-VC and comparison vaccines (Figures 5–9).
Outcomes for a similar JE vaccine in children
In addition to the studies of JE-VC, we reviewed evidence for serious adverse events and adverse events of special interest (fever, rash) in one RCT performed using a similar JE vaccine (JEEV) among children aged 1 and 2 years in India [Biological E 2013] (Table 3). The findings were for this vaccine were similar to those seen with JE-VC.
Additional information
The results described here include all reported adverse events, whether assessed as related or unrelated. Causality often cannot be determined, especially in surveillance data when reported events occur among persons who often have received multiple vaccines concurrently.
Conclusion
On the basis of these adverse event and safety data from clinical trials and surveillance, the work group concluded the undesirable anticipated effects were small.
Criterion 3: Balance of desirable versus undesirable effects
Criterion question: Do the desirable effects outweigh the undesirable effects?
JE-VC is the only JE vaccine licensed and available in the United States. With no alternative vaccine, this assessment was focused on comparing the balance of risks and benefits of JE-VC. Seroprotection rates were high at both 1 month and 5 to 6 months after the 2-dose primary series of JE-VC. JE-VC seroprotection rates were non-inferior to rates for other JE vaccines previously available in the United States or used internationally.
Serious adverse events following JE-VC were uncommon within 1 month and 6 to 7 months of vaccination. In RCTs, there were no significant differences in the proportions of subjects with serious adverse events occurring within 1 month or 6 to 7 months in the JE-VC or comparison groups. Comparator groups in the largest studies received the commonly used 7-valent pneumococcal conjugate vaccine (Prevnar) or hepatitis A vaccine (Havrix 720), or phosphate buffered saline with 0.1% aluminum hydroxide. For adverse events of special interest including fever, rash, hypersensitivity/urticaria, neurologic, and medically attended adverse events, there also were no significant differences in the proportions with any of these events among JE-VC and comparator vaccine recipients. Post-marketing surveillance data from >1.3 million doses distributed provided additional reassuring data, with reported rates of serious adverse events similar to rates reported in post-marketing surveillance assessments for other vaccines used in the United States. No patterns in the timing or types of serious adverse events were identified in the clinical trials or surveillance data. Overall, no important safety concerns were identified.
Conclusion
In general, with high seroprotection rates following vaccination and with no important safety concerns identified, the work group considered the desirable effects of a vaccine to prevent a rare but potentially serious, untreatable disease outweighed the undesirable effects of vaccination. However, as with any vaccine, rare serious adverse events can occur, and so for some travelers with lower risk itineraries, even a low probability of vaccine-related serious adverse events might be higher than the risk for disease. Therefore, for each traveler, a healthcare provider should consider and discuss the balance of desirable and undesirable vaccine effects and the traveler’s probable risk based on itinerary and activities, and JE vaccine should be targeted to travelers who are at higher risk for disease.
Criterion 4: Certainty of evidence for outcomes
Criterion question: What is the overall certainty of this evidence for the critical outcomes?
The GRADE approach was followed for assessing the type of evidence [Ahmed 2013]. For the benefits considered critical outcomes (i.e., seroprotection at 1 month and 5 to 6 months following JE-VC), evidence type is 1 for the RCTs and 3 for observational studies (Table 15). For harms considered critical outcomes (i.e., serious adverse events and adverse events of special interest) evidence type is 2 for RCTs (downgraded because of inadequate blinding) and 3 for observational studies.
Additional information
For serious adverse events at 1 month, there was some concern about inconsistency and imprecision in the RCTs. However, complementary information from surveillance assessments and observational studies provided additional data supportive of the vaccine’s safety.
Conclusion
The overall quality of evidence is type 1 (i.e., high) for vaccine effectiveness using seroprotection as the endpoint and type 2 (i.e., moderate) for safety (Table 16).
Domain 3. Values
Criterion 1: Target population perception of value
Criterion question: Does the target population feel that the desirable effects are large relative to undesirable effects?
A population survey was conducted using the Porter Novelli Public Services Styles survey mechanism to gather data on perspectives and perceptions of JE disease and the vaccine. Porter Novelli maintains a panel of approximately 55,000 persons representative of the non-institutionalized U.S. population. Members are randomly recruited by mail using probability-based sampling by address. Respondents receive cash-equivalent reward points for their participation. Porter Novelli regularly conducts surveys covering a variety of topics. For the SpringStyles survey conducted from March 21 through April 11, 2018, 10,904 adults were invited to participate.
Two JE questions were included in the survey:
- You are going on a trip to another country. You have a one in a million chance of getting a disease. About one-third of people who get the disease will die and one-third of people will have a permanent disability like problems with walking or thinking clearly. There is a vaccine that prevents the disease. It is safe although on rare occasions can cause serious side effects. It costs $600 and is not covered by insurance. How likely are you to get the vaccine?
- Possible responses: Very likely/Somewhat likely/Not sure/Somewhat unlikely/Very unlikely
- Which factors were most important in deciding whether you would get the vaccine? (One or more factors can be selected)
- Possible responses: Chance of getting the disease/Chance of dying or being disabled/The vaccine is safe/Chance of serious side effects/Cost of the vaccine/I do not get any vaccinations/Other reasons not listed
In total, 6,427 (59%) of invited participants completed the survey. Median age was 51 years (range: 18–94 years) and 2,907 (45%) were male. Races included White, Non-Hispanic (n=4,796; 74%), Hispanic (n=675; 11%), Black, Non-Hispanic (n=547; 9%) and other (n=436; 7%). There were 328 (5%) with no formal education, 1,456 (23%) with up to high school education, and 4,643 (72%) with at least some college or higher education. Household income was <$20,000 for 5,967 (9%), $25,000 to <$60,000 for 1,890 (29%), and ≥$60,000 for 3,940 (61%). Data were weighted using eight factors including gender, age, household income, race/ethnicity, household size, education, census region, and metro status to match U.S. Current Population Survey proportions.
Responses to the JE questions were provided by 6,384 (99%) of 6,427 participants. Among respondents, the likelihood of getting JE vaccine was “very likely” for 1,032 (16%), “somewhat likely” for 1,033 (16%), “not sure” for 1,575 (25%), “somewhat unlikely” for 1,088 (17%) and “very unlikely” for 1,656 (26%). Overall, there were more unlikely to get vaccinated (43%) compared with those likely to get vaccinated (32%).
Conclusion
The results suggest there is variability in the population perception of whether the potential benefits of vaccination outweigh harms.
Criterion 2: Uncertainty around target population perception of value
Criterion question: Is there important uncertainty about or variability in how much people value the main outcomes?
Among the 2,065 respondents “very likely” or “somewhat likely” to get the vaccine, the three most important factors for their decision were chance of getting the disease (n=1,439; 70%), the chance of dying or being disabled (n=1,209; 59%) and that the vaccine is safe (n=847; 41%). Compared with those “very likely” to get the vaccine, those “somewhat likely” to get the vaccine more frequently noted vaccine cost (35% versus 10%) and the chance of serious side effects (26% versus 16%). Among the 2,744 “very unlikely” or “somewhat unlikely” to get the vaccine, the three most important factors were cost of the vaccine (n=1,771; 65%), chance of getting the disease (1,281; 47%), and chance of serious side effects (n=756; 28%). Of note, among those “very likely” to get the vaccine, 76% selected the chance of getting the disease as an important factor in their decision, and among those “very unlikely” to get the vaccine, 41% also selected the chance of getting the disease as a factor.
Conclusion
The survey results suggest there are differences in the population regarding perceptions of disease risk and the value of vaccination. While some in the population clearly value the availability of a vaccine to prevent a rare disease with potentially serious outcomes and no specific treatment, others were less likely to place value on it when the vaccine is expensive and, while generally safe, has the possibility of rare serious side effects. Disease risk was considered a reason to both receive and not receive the vaccine. There is clearly substantial variability in individual perception and tolerance of risk impacting decision-making on vaccination choices.
Domain 4. Acceptability
Criterion: Acceptability to key stakeholders
Criterion question: Is the option acceptable to key stakeholders?
Travel medicine practitioners were considered an important stakeholder group in regards to the use of JE vaccine. The work group investigated mechanisms to survey U.S. travel medicine practitioners regarding JE vaccine recommendations but none could be identified within the timeframe available. Four members of the JE ACIP work group who are travel medicine practitioners and members of the International Society of Travel Medicine played an active role in discussions about the recommendations.
Several publications authored by U.S. healthcare providers have included opinions on the existing ACIP JE vaccine recommendations [Caldwell 2018, Connor 2017, Burchard 2009, Teitelbaum 2009, Shlim 2002]. The opinions on JE vaccination expressed in these publications and in meetings range from suggesting limited use based on an individual assessment for each traveler to broader consideration for any traveler to a rural or peri-urban area irrespective of duration of travel or itinerary.
The manufacturer of Ixiaro (Valneva) sponsored an “Expert Advisory Group on JE Prevention” that has had several meetings and written three letters to ACIP in January 2015, April 2017, and October 2017. The group has suggested revisions to the JE vaccine recommendations and urged broadening of the recommendations to include traveler groups the work group considered ill-defined for being at higher risk. The work group reviewed the letters and two of the travel medicine practitioners with dissenting opinions on the recommendations made presentations to the work group. However, it is unknown how representative their opinions are among other travel medicine practitioners. The manufacturer has similarly suggested broadening of the recommendations, but due to a conflict of interest, acceptability to the manufacturer was given low priority.
For the public, the proposed recommendations for JE vaccination are likely to be acceptable as they describe and target vaccination of travelers with the highest risk for infection and so limit the number of travelers for whom an expensive vaccine for a very low risk disease might be recommended. Factors that increase risk for JE among travelers are described in the JE vaccine recommendations. Most travelers are likely to value a discussion of the risks and benefits of vaccination including consideration of the traveler’s tolerance of risk, while others might prefer more concrete recommendations that clearly define who should receive vaccine based on a specific factor such as duration of travel. Some travelers who have an insurer that covers travel vaccines might prefer a recommendation for vaccination for all travelers.
Conclusion
While there is some variability among stakeholders opinions on the recommendations, there is stakeholder agreement that: 1) There is overall low risk for most travelers; 2) There is a need to inform travelers about risks and prevention measures for JE; and 3) Vaccine should be targeted to travelers at higher risk. All members of the work group agreed the vaccine recommendations were acceptable, and considered that they will probably be acceptable to most stakeholders as they are based on individual clinical decision-making with consideration of 1) risks related to the specific travel itinerary, 2) likelihood of future travel to JE-endemic countries, 3) high morbidity and mortality of JE when it occurs, 4) availability of an effective vaccine, 5) possibility, but low probability, of serious adverse events following vaccination, and 6) traveler’s personal perception and tolerance of risk.
Domain 5. Resource Use
Criterion: Resource allocation
Criterion question: Is the option a reasonable and efficient allocation of resources?
JE vaccination is cost-effective or cost-saving for local populations in JE endemic countries [Yin 2012, Touch 2010, Siraprapasiri 1997]. However, JE vaccination is not expected to be cost effective among travelers as there is 1) a substantially lower risk of disease of <1 reported case per million U.S. travelers compared with 1–10 cases of JE per 100,000 population per year in endemic countries, and 2) use of much lower cost vaccines in most vaccination programs in Asia (e.g., a live attenuated JE vaccine manufactured in China and used widely throughout Asia costs <1 USD per dose compared with a 2-dose primary series of JE-VC for US travelers which costs approximately 600 USD).
There were several general resource considerations the work group noted when discussing JE vaccination for travelers. From a societal perspective, JE vaccination is probably not an efficient use of resources. The vaccine is expensive and the disease is rare. However, the question of resource use is less relevant for travel vaccines which are usually paid for by the travelers themselves and are not covered under the Vaccines for Children program or by most insurance plans. Travelers make individual decisions on vaccination. Mortality and disability rates following disease are high, and about one third of participants in the survey described in Domain 3 indicated vaccination was probably a reasonable investment. Nonetheless, there are opportunity costs in travelers purchasing this vaccine compared with an alternative preventive measure. The cost of the vaccine also raises a health equity issue, and could lead to health disparities since some higher risk travelers might not be able to afford the vaccine; however, the vaccine recommendations cannot address this issue.
The work group did not consider a cost-effectiveness analysis of JE-VC essential. However, the work group decided to perform a comparative analysis of different vaccination strategies to: 1) Provide perspective on the numbers of travelers needed to be vaccinated and associated costs to avert a case; 2) Compare the relative costs of vaccination for travelers with different itineraries and disease risk; and 3) Better understand the cost implications of possibly expanding the current JE vaccine recommendations to a broader group of travelers. A comparative analysis of strategies for JE vaccination for U.S. travelers to Asia was performed by CDC’s Health Economics and Modeling Unit according to ACIP guidelines [Meltzer 2018]. An analytic horizon of 6 years was used, but productivity losses were evaluated over average life expectancy. The analysis compared JE vaccination in three groups. Risk group l included travelers who plan to spend ≥1 month in JE endemic areas and approximates the group for whom JE vaccine is recommended under ACIP guidelines. Risk group 2 included travelers who will spend <1 month in JE endemic areas with at least 20% of their time doing outdoor activities in rural areas. This group approximates travelers for whom JE vaccination should be considered after evaluating their itinerary and weighing the benefits, risks, and costs. Risk group 3 included the remainder of shorter-term and lower-risk U.S. travelers to Asia for whom JE vaccination is not recommended.
To prevent one JE case, the number of travelers who would need to be vaccinated was 0.7 million in Risk group 1, 1.6 million in Risk group 2, and 9.8 million in Risk group 3. The cost to prevent one JE case from a societal perspective was approximately $0.6 billion for Risk group 1, $1.3 billion for Risk group 2, and $7.9 billion for Risk group 3. The variable with the greatest influence on the cost-effectiveness of vaccination was disease incidence among travelers. As baseline incidence relies upon reported JE cases, to address any uncertainty about the sensitivity of surveillance, a sensitivity analysis was conducted increasing the baseline incidence 100 times, although it is very unlikely 100 JE cases are occurring among U.S. travelers annually when fewer than 1 case is reported. With the incidence 100 times higher, the numbers needed to vaccinate to prevent a case were 7,000, 16,000 and 98,000, and the cost per case averted was $5 million, $12 million, and $78 million in each Risk group, respectively. If JE vaccination recommendations were expanded from Risk group 1 to Risk group 1 and 2, it would cost society an additional $1.6 billion to prevent one additional case of JE. Similarly, expanding JE vaccination recommendations from Risk groups 1 and 2 to all travelers would cost society an additional $14.6 billion to prevent an additional JE case.
Between 1993 and 2017, among 12 JE cases reported in US travelers, 8 (67%) occurred among the 20% travelers in the category in which vaccination would have been recommended. Among the remainder of travelers for whom itinerary information was known (n=3), all had at least some rural exposure which would have warranted discussion of risks, prevention measures, and consideration of vaccination [Hills 2017].
Conclusion
The work group decided the question of whether the intervention was a reasonable and efficient allocation of collective resources was not directly applicable to JE vaccination as travel vaccines are usually paid for by the travelers themselves who make individual decisions on vaccine purchase. In general, JE vaccination for travelers cannot be considered an efficient use of societal resources as it is an expensive vaccine for a low risk disease in this population. Nonetheless, the comparative analysis supports the proposed tiered JE vaccine recommendations as it indicated a large increased cost to society to prevent a case of JE when including Risk groups 1 and 2 compared with Risk group 1 alone, supporting a more cautious approach or “consideration” of vaccination for those in Risk group 2. In addition, there was a very large increased cost to society if Risk group 3 was included, which does not support a broad recommendation of JE vaccination for all travelers. Overall, vaccine recommendations targeted to higher risk groups are probably a reasonable allocation of resources as the financial implications of vaccine purchase will be borne by travelers most at risk of a severe disease who will therefore receive the most benefit.
Domain 6
Criterion: Implementation feasibility
Criterion question: Is the option feasible to implement?
JE vaccination is provided by generalist and specialist healthcare providers. Administration is feasible as part of a pre-travel consultation. Barriers to implementation of risk-based vaccine recommendations include lack of understanding of factors that might increase the risk for JE and, therefore, which travelers might benefit most from vaccination. However, specific information is provided in a table accompanying the recommendations that guides practitioners on factors that increase JE risk (Box). Other resources such as the CDC Yellow Book: Health Information for International Travel also are readily available [CDC 2017].
Conclusion
The work group considered risk-based recommendations were probably feasible to implement.
Conclusions and Additional Considerations
Conclusions
Overall, the work group determined the desirable consequences probably outweigh undesirable consequences in most settings when risk-based recommendations are appropriately implemented. We recommend the intervention for individuals based on clinical decision-making. The proposed recommendations are presented below.
Additional considerations
Among the shorter-term travelers for whom vaccination would be “considered” rather than “recommended” there was no consistent risk factor, destination, or feature to enable further targeting of recommendations. This suggests the only way to prevent every case would be to recommend vaccination for all travelers; however, weighing the risks and benefits, the work group members considered vaccine recommendations should be targeted to the subset of travelers with greater risk of infection.
Recommendations for the Prevention of JE Among Travelers
JE is a very low risk disease for most U.S. travelers to JE-endemic countries. However, some travelers will be at increased risk of infection based on their planned itinerary. Factors that increase the risk of JE virus exposure include: 1) longer duration of travel, 2) travel during the JE virus transmission season, 3) spending time in rural areas, 4) participating in extensive outdoor activities, and 5) staying in accommodations without air conditioning, screens, or bed nets (Box).
Healthcare providers should assess each traveler’s risk for mosquito exposure and JE virus infection based on their planned itinerary, and discuss ways to reduce their risk. All travelers to JE-endemic countries should be advised to take precautions to avoid mosquito bites to reduce the risk for JE and other vector-borne diseases. These precautions include using insect repellent, permethrin-impregnated clothing, and bed nets, and staying in accommodations with screened or air-conditioned rooms.
For some people who might be at increased risk for JE based on travel duration, season, location, activities, and accommodations, JE vaccine can further reduce the risk for infection. The decision whether to vaccinate should be individualized and consider the: 1) risks related to the specific travel itinerary, 2) likelihood of future travel to JE-endemic countries, 3) high morbidity and mortality of JE when it occurs, 4) availability of an effective vaccine, 5) possibility, but low probability, of serious adverse events following vaccination, and 6) traveler’s personal perception and tolerance of risk.
JE vaccine is recommended for persons moving to a JE-endemic country to take up residence, longer-term (e.g., ≥1 month) travelers to JE-endemic areas, and frequent travelers to JE-endemic areas. JE vaccine also should be considered for shorter-term (e.g., <1 month) travelers with an increased risk of JE based on planned travel duration, season, location, activities, and accommodations (Box). Vaccination also should be considered for travelers to endemic areas who are uncertain of specific duration of travel, destinations, or activities.
JE vaccine is not recommended for travelers with very low risk itineraries, such as shorter-term travel limited to urban areas or travel that occurs outside of a well-defined JE virus transmission season.
Box. Factors that increase risk for Japanese encephalitis among travelers
Duration
- Highest incidence of disease has been reported among longer-term travelers.
- Although no specific duration of travel puts a traveler at risk for JE, longer-term travel increases the likelihood that a traveler might be exposed to an infected mosquito.
- Longer-term travel includes cumulative periods in endemic areas; this includes frequent travelers, and persons residing in urban areas who are likely to visit higher risk rural areas.
Season
- JE virus transmission occurs seasonally in some areas, and year-round in other areas.
- Information on expected JE virus transmission by country is available on the CDC website (see Japanese encephalitis chapter in CDC Health Information for International Travel [the Yellow Book]). These data should be interpreted cautiously because JE virus transmission varies within countries and from year to year.
Location
- Highest risk occurs from mosquito exposure in rural or agricultural areas.
- Mosquitoes that transmit JE virus typically breed in flooded rice fields, marshes, and other stagnant collections of water.
- Some cases have been reported among travelers to coastal areas or resorts located in or adjacent to rural or rice growing areas.
- JE can occur in large, focal outbreaks indicating extensive active JE virus transmission in those areas.
Activities
- The mosquitoes that transmit JE virus feed most often in the outdoors, particularly from sunset through dawn, so examples of activities that increase risk include:
- Outdoor recreation such as camping, hiking, trekking, biking, rafting, fishing, hunting, or farming.
- Spending substantial time outdoors, especially during the evening or night.
Accommodations
Accommodations without air conditioning, screens, or bed nets increase risk of mosquito exposure.
References
-
- ACIP. Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) for Use of Inactivated Vero Cell Culture-derived Japanese Encephalitis Vaccine in Children. 2013. Accessed April 11, 2018
- Ahmed F. U.S. Advisory Committee on Immunization Practices handbook for developing evidence-based recommendations [52 pages]. Version 1.2. Atlanta, GA: Centers for Disease Control and Prevention (CDC); 2013.
- Arana JE, Harrington T, Cano M, et al. Post-licensure safety monitoring of quadrivalent human papillomavirus vaccine in the Vaccine Adverse Event Reporting System (VAERS), 2009-2015. Vaccine. 2018;36:1781–8.
- Biological E. Unpublished data presented to the ACIP JE Vaccine Work Group by Katrin Dubischar-Kastner (Intercell AG, now Valneva Austria GmbH). April 2013.
- Burchard GD, Caumes E, Connor BA, et al. Expert opinion on vaccination of travelers against Japanese encephalitis. J Travel Med. 2009;16:204–16.
- Butler S, Sutter D, Maranich A. Tolerability of Japanese encephalitis vaccine in pediatric patients. J Pediatric Infect Dis Soc. 2017;6:149–152.
- Caldwell JP, Chen LH, Hamer DH. Evolving epidemiology of Japanese encephalitis: implications for vaccination. Curr Infect Dis Rep. 2018;20:30.
- Campbell GL, Hills SL, Fischer M, e al. Estimated global incidence of Japanese encephalitis: a systematic review. Bull World Health Organ. 2011;89:766–74.
- CDC. Recommendations for use of a booster dose of inactivated Vero cell culture-derived Japanese encephalitis vaccine: Advisory Committee on Immunization Practices, 2011. MMWR. 2011;60:661–3.
- CDC. CDC Yellow Book 2018: Health Information for International Travel. New York: Oxford University Press; 2017.
- Central Drugs Standard Control Organization. Product approval information for JEEV (Japanese encephalitis purified inactivated vaccine adsorbed). Biological E Limited, Hyderabad, India. 2013.
- Connor B, Bunn W. The changing epidemiology of Japanese encephalitis and new data: The implications for new recommendations for Japanese encephalitis vaccine. Trop Dis Travel Med Vaccines. 2017;3:14.
- Cramer JP, Dubischar K, Eder S, et al. Immunogenicity and safety of the inactivated Japanese encephalitis vaccine IXIARO® in elderly subjects: Open-label, uncontrolled, multi-center, phase 4 study. Vaccine. 2016(a);34:4579–85.
- Cramer JP, Jelinek T, Paulke-Korinek M, et al. One-year immunogenicity kinetics and safety of a purified chick embryo cell rabies vaccine and an inactivated Vero cell-derived Japanese encephalitis vaccine administered concomitantly according to a new, 1-week, accelerated primary series. J Travel Med. 2016(b);23(3).
- Dubischar-Kastner K, Eder S, Buerger V, et al. Long-term immunity and immune response to a booster dose following vaccination with the inactivated Japanese encephalitis vaccine IXIARO, IC51. Vaccine. 2010(a);28:5197–202.
- Dubischar-Kastner K, Kaltenboeck A, Klingler A, et al. Safety analysis of a Vero-cell culture derived Japanese encephalitis vaccine, IXIARO® (IC51), in 6 months of follow-up. Vaccine. 2010(b);28:6463–9.
- Dubischar KL, Kadlecek V, Sablan JB, et al. Immunogenicity of the inactivated Japanese encephalitis virus vaccine IXIARO in children from a Japanese encephalitis virus-endemic region. Pediatr Infect Dis J. 2017(a);36:898–904.
- Dubischar KL, Kadlecek V, Sablan B et al. Safety of the inactivated Japanese encephalitis virus vaccine IXIARO in children: An open-label, randomized, active-controlled, phase 3 study. Pediatr Infect Dis J. 2017(b);36:889–97.
- Erra EO, Hervius Askling H, Rombo L, et al. A single dose of Vero cell–derived Japanese encephalitis (JE) vaccine (IXIARO) effectively boosts immunity in travelers primed with mouse brain–derived JE vaccines. Clin Infect Dis. 2012;55:825–34.
- Food and Drug Administration. CFR – Code of Federal Regulations 21CFR312.32. Accessed October 12, 2018.
- Fischer M, Lindsey N, Staples JE, Hills S. Japanese encephalitis vaccines: Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR. 2010;59(RR-1):1-27.
- Hills S. Epidemiology and risk of Japanese encephalitis in U.S. travelers. Presentation to the Advisory Committee on Immunization Practices (ACIP), October 26, 2017, Atlanta, GA. Accessed September 25, 2018.
- Hombach J, Solomon T, Kurane I, et al. Report on a WHO consultation on immunological endpoints for evaluation of new Japanese encephalitis vaccines, WHO, Geneva, 2–3 September, 2004. Vaccine. 2005;23:5205–11.
- Jelinek T, Burchard GD, Dieckmann S et al. Short-term immunogenicity and safety of an accelerated pre-exposure prophylaxis regimen with Japanese encephalitis vaccine in combination with a rabies vaccine: A phase III, multicenter, observer-blind study. J Travel Med. 2015(a);22:225–31.
- Jelinek T, Cramer JP, Dieckmann S, et al. Evaluation of rabies immunogenicity and tolerability following a purified chick embryo cell rabies vaccine administered concomitantly with a Japanese encephalitis vaccine. Travel Med Infect Dis. 2015(b);13:241–50.
- Jelinek T, Cromer MA, Cramer JP, et al. Safety and immunogenicity of an inactivated Vero cell-derived Japanese encephalitis vaccine (IXIARO®, JESPECT®) in a pediatric population in JE non-endemic countries: An uncontrolled, open-label phase 3 study. Travel Med Infect Dis. 2018;22:18–24.
- Kaltenböck A, Dubischar-Kastner K, Eder G, et al. Safety and immunogenicity of concomitant vaccination with the cell-culture based Japanese encephalitis vaccine IC51 and the hepatitis vaccine HAVRIX1440 in healthy subjects—A single-blind, randomized, controlled phase 3 study. Vaccine. 2009;27:4483–9.
- Kaltenböck A, Dubischar-Kastner K, Schuller E, et al. Immunogenicity and safety of IXIARO (IC51) in a phase II study in healthy Indian children between 1 and 3 years of age. Vaccine. 2010;28:834–9.
- Lee G, Carr W. Updated framework for development of evidence-based recommendations by the Advisory Committee on Immunization Practices. MMWR Morb Mortal Wkly Rep. 2018;67:103–8.
- Lindsey NP, Rabe IB, Miller ER et al. Adverse event reports following yellow fever vaccination, 2007– Travel Med. 2016;23:1–6.
- Lyons A, Kanesa-thasan N, Kuschner RA, et al. A Phase 2 study of a purified, inactivated virus vaccine to prevent Japanese encephalitis. Vaccine. 2007;25:3445–53.
- Markoff L. Points to consider in the development of a surrogate for efficacy of novel Japanese encephalitis virus vaccines. Vaccine. 2000;18 Suppl 2:26–32.
- Meltzer M. Comparative analysis of strategies for JE vaccination for U.S. travelers to Asia. Presentation to the Advisory Committee on Immunization Practices (ACIP), June 21, 2018, Atlanta, GA. Accessed September 20, 2018.
- Miller ER, Moro PL, Cano M, et al. Post-licensure safety surveillance of 23-valent pneumococcal polysaccharide vaccine in the Vaccine Adverse Event Reporting System (VAERS), 1990-2013. Vaccine. 2016;34:2841–6.
- Miller ER, Lewis P, Shimabukuro TT, et al. Post-licensure safety surveillance of zoster vaccine live (Zostavax®) in the United States, Vaccine Adverse Event Reporting System (VAERS), 2006-2015. Hum Vaccin Immunother. 2018; 14:1963–9.
- Rabe IB, Miller ER, Fischer M, Hills SL. Adverse events following vaccination with an inactivated, Vero cell culture-derived Japanese encephalitis vaccine in the United States, 2009-2012. Vaccine. 2015;33:708–12.
- Schuller E, Jilma B, Voicu V, et al. Long-term immunogenicity of the new Vero cell-derived, inactivated Japanese encephalitis virus vaccine IC51 Six and 12 month results of a multicenter follow-up phase 3 study. Vaccine. 2008;26:4382–6.
- Schuller E, Klade CS, Wolfl G, et al. Comparison of a single, high-dose vaccination regimen to the standard regimen for the investigational Japanese encephalitis vaccine, IC51: A randomized, observer-blind, controlled Phase 3 study. Vaccine. 2009;27:2188–93.
- Schuller E, Klingler A, Dubischar-Kastner K, et al. Safety profile of the Vero cell-derived Japanese encephalitis virus (JEV) vaccine IXIARO. Vaccine. 2011;29:8669–76.
- Shlim D, Solomon T. Japanese encephalitis vaccine for travelers: Exploring the limits of risk. Clin Infect Dis. 2002;35:183–8.
- Siraprapasiri T, Sawaddiwudhipong W, Rojanasuphot S. Cost benefit analysis of Japanese encephalitis vaccination program in Thailand. Southeast Asian J Trop Med Public Health. 1997;28:143–8.
- Tauber E, Kollaritsch H, Korinek M, et al. Safety and immunogenicity of a Vero-cell-derived, inactivated Japanese encephalitis vaccine: A non-inferiority, phase III, randomised controlled trial. Lancet. 2007;370:1847–53.
- Tauber E, Kollaritsch H, von Sonnenburg F, et al. Randomized, double-blind, placebo-controlled phase 3 trial of the safety and tolerability of IC51, an inactivated Japanese encephalitis vaccine. J Infect Dis. 2008;198:493–9.
- Taucher 2017. VLA-401: JE-VC post-marketing adverse event surveillance among U.S. military personnel. Presentation to the Advisory Committee on Immunization Practices (ACIP), October 26, 2017, Atlanta, GA.
- Teitelbaum P. Expert opinion on vaccination of travelers against Japanese encephalitis. J Travel Med. 2009;16:441.
- Touch S, Suraratdecha C, Samnang C, et al. A cost-effectiveness analysis of Japanese encephalitis vaccine in Cambodia. Vaccine. 2010;28:4593–9.
- Walker WL, Hills SL, Miller ER, et al. Adverse events following vaccination with an inactivated, Vero cell culture-derived Japanese encephalitis vaccine in the United States, 2012–2016. Vaccine. 2018;36:4369–74.
- Woolpert T, Staples JE, Faix DJ, et al. Immunogenicity of one dose of Vero cell culture-derived Japanese encephalitis (JE) vaccine in adults previously vaccinated with mouse brain-derived JE vaccine. Vaccine. 2012;30:3090–6.
- Yin Z, Beeler Asay GR, Zhang L, et al. An economic evaluation of the use of Japanese encephalitis vaccine in the expanded program of immunization of Guizhou province, China. Vaccine. 2012;30:5569–77.
Tables
Table 1. Summary of outcome measure ranking and inclusion for use of inactivated Vero cell culture-derived JE vaccine (JE-VC)
| Outcome | Importance | Data available | Include in evidence profile |
|---|---|---|---|
| Benefits | |||
| Vaccine efficacy to prevent JE disease | Critical | No | No |
| Seroprotection at 1 month after primary series[1] | Critical | Yes | Yes |
| Seroprotection at 6 months after primary series[1] | Critical | Yes | Yes |
| Harms | |||
| Serious adverse events[2] | Critical | Yes | Yes |
| Adverse events of special interest[3] | Critical | Yes | Yes |
| Injection site reactions | Important | — | No |
| Interference with response to other vaccines | Important | — | No |
Table 1 Footnotes
JE=Japanese encephalitis
- Seroprotection defined as a neutralizing antibody titer ≥10 by 50% plaque reduction neutralization test against the JE virus SA14-14-2 JE virus [Markoff 2000; Hombach 2005].
- Serious adverse event defined as any of the following outcomes: 1) death, 2) life-threatening adverse event, 3) inpatient hospitalization, 4) persistent or significant incapacity or substantial disruption of the ability to conduct normal life functions, or 5) congenital anomaly/birth defect [FDA. 21 CFR 312.32].
- Systemic adverse events evaluated include fever, rash, hypersensitivity/urticaria, neurologic, and medically attended adverse events.
Table 2. Seroprotection at 1 month after a 2-dose primary series of inactivated Vero cell culture-derived JE vaccine (JE-VC) administered according to the FDA-approved dose and schedule
| Study | Sites | Type | Age group | PRNT50 titer ≥10 | |||
|---|---|---|---|---|---|---|---|
| JE-VC | Other JE vaccine[1] |
||||||
| No. | (%) | No. | (%) | ||||
| Kaltenbock 2010 | India | RCT | Children (1-2 yrs) | 22/23 | (96)[2] | 10/11 | (91) |
| Tauber 2007 | U.S./Europe | RCT | Adults (≥18 yrs) | 352/361 | (98) | 347/364 | (95) |
| Lyons 2007 | U.S. | RCT | Adults (18–49 yrs) | 21/22 | (95) | 14/19 | (74) |
| Biological E 2013 | India | RCT | Adults (18–49 yrs) | 53/54 | (98) | 107/108 | (99) |
| Dubischar 2017(a) | Philippines | Obs[3] | Children (2 mos-17 yrs) | 384/385 | (99)[4] | — | — |
| Jelinek 2018 | U.S./Europe/Australia | Obs | Children (2 mos-17 yrs) | 62/62 | (100) | — | — |
| Schuller 2009 | Europe | Obs[3] | Adults (≥18 yrs) | 110/113 | (97) | — | — |
| Kaltenbock 2009 | Europe | Obs[3] | Adults (≥18 yrs) | 126/127 | (99) | — | — |
| Woolpert 2012 | U.S. | Obs | Adults (≥18 yrs) | 88/92 | (96) | — | — |
| Erra 2012[5] | Europe | Obs | Adults (≥18 yrs) | 30/31 | (97) | 13/15 | (87) |
| Jelinek 2015(a) | Europe | Obs[3] | Adults (18–65 yrs) | 206/206 | (100)[6] | — | — |
| Cramer 2016(a)[7] | Europe | Obs | Adults (64–83 yrs) | 197128/ | (65) | — | — |
Table 2 Footnotes
JE=Japanese encephalitis; PRNT50=50% plaque reduction neutralization test; RCT=Randomized controlled trial; Obs=Observational study
- Inactivated mouse brain-derived JE vaccine (JenceVac) manufactured by Green Cross (Korea) [Kaltenbock 2010; Erra 2012], inactivated mouse brain-derived JE vaccine (JE-VAX) manufactured by Biken (Japan) [Tauber 2007; Lyons 2007], or inactivated Vero cell culture-derived JE vaccine adsorbed (JEEV) manufactured by Biological E (India) [Biological E 2013(a)].
- Of an additional 21 children aged 1-2 years who received two 0.5mL doses of JE-VC, 20 (95%) were seroprotected at 1 month after the second dose.
- RCT with no comparative immunogenicity data.
- Of an additional 98 children aged 3-11 years who received two 0.25mL doses of JE-VC, 94 (96%) were seroprotected at 1 month after the second dose.
- Seroprotection measured at 4–8 weeks after final vaccine dose.
- Complete data unavailable and estimated from graph.
- Seroprotection measured at 42 days after dose 2 of JE-VC.
Figure 1. Pooled risk ratio for seroprotection at 1 month after a 2-dose primary series of inactivated Vero cell culture-derived Japanese encephalitis vaccine (JE-VC) in randomized controlled trials*
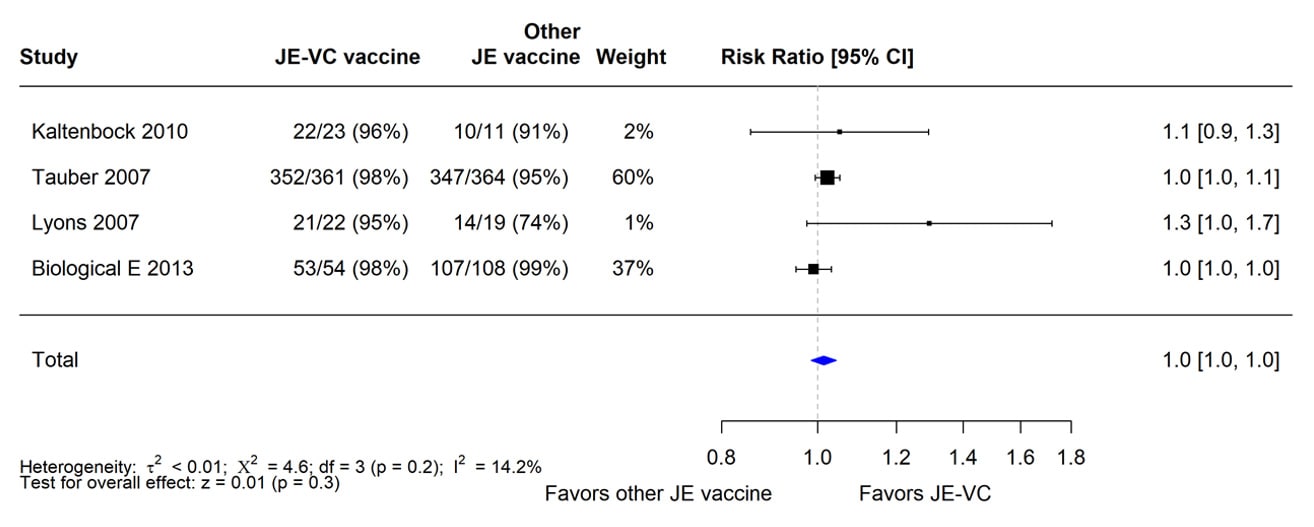
*Pooled risk ratios computed using the random effects model (Mantel-Haenszel method). Risk ratio = Proportion seroprotected in JE-VC group / Proportion seroprotected in other Japanese encephalitis (JE) vaccine group. Risk ratio >1.0 favors JE-VC versus other JE vaccine.
Table 3. Seroprotection, serious adverse events, and systemic adverse events following receipt of JEEV[1,2]
| Outcome | JEEV | JenceVac[3] | ||
|---|---|---|---|---|
| No. | (%) | No. | (%) | |
| Seroprotection (PRNT50 titer ≥10) at 1 month after a 2-dose primary series | 258/280 | (92) | 140/142 | (99) |
| Serious adverse events within 1 month of any dose | 1/304 | (<1) | 1/152 | (1) |
| Solicited systemic adverse events within 7 days after any dose | ||||
|
34/304 | (11) | 24/152 | (16) |
|
4/304 | (1) | 2/152 | (1) |
Table 3 Footnotes
- JEEV is manufactured by Biological E (Hyderabad, India) with technology licensed from Intercell (now Valneva Austria GmbH). JEEV and JE-VC (IXIARO) use the same virus strain, adjuvant, and virus purification; however, no process comparability studies have been completed and it cannot be assumed that the two final vaccine products are the same [Central Drugs Standard Control Organization 2013].
- Randomized, controlled, open-label study in India in which children aged 1–2 years received two 0.25mL doses of JEEV (N=304) or three doses of JenceVac (N=152). The seroprotection rate in JEEV recipients was non-inferior to that in JenceVac recipients [Biological E 2013].
- Inactivated mouse brain-derived JE vaccine (JenceVac) manufactured by Green Cross (Korea).
Table 4. Seroprotection at 5 to 6 months after a 2-dose primary series of inactivated Vero cell culture-derived JE vaccine (JE-VC) administered according to the FDA-approved dose and schedule[1]
| Study | Sites | Type | Age group | PRNT50 titer ≥10 | |||
|---|---|---|---|---|---|---|---|
| JE-VC | Other JE vaccine[2] |
||||||
| No. | (%) | No. | (%) | ||||
| Lyons 2007 | U.S. | RCT | Adults (18–49 yrs) | 17/17 | (100) | 7/13 | (54) |
| Schuller 2008 | Europe | RCT | Adults (≥18 yrs) | 172/181 | (95) | 61/82 | (74) |
| Dubischar 2017(a) | Philippines | Obs[3] | Children (2 mos–17 yrs) | 358/389 | (92)[4] | — | — |
| Jelinek 2018 | U.S./Europe/Australia | Obs | Children (2 mos–17 yrs) | 31/34 | (91) | — | — |
| Dubischar-Kastner 2010 (a) | Europe | Obs | Adults (≥18 yrs) | 96/116 | (83) | — | — |
| Cramer 2016(b) | Europe | Obs[3] | Adults (18–65 yrs) | 190/204 | (93) | — | — |
Table 4 Footnotes
JE=Japanese encephalitis; PRNT50=50% plaque reduction neutralization test; RCT=Randomized controlled trial; Obs=Observational study
- For studies in children, follow-up was at 6 months after completing the 2-dose primary series. For studies in adults, follow-up was at 5 months after completing the 2-dose primary series or 6 months after the first dose.
- Inactivated mouse brain-derived JE vaccine (JE-VAX) manufactured by Biken (Japan).
- RCT with no comparative immunogenicity data.
- Of an additional 96 children aged 3-11 years who received two 0.25mL doses of JE-VC, 74 (77%) were seroprotected at 6 months after the second dose.
Figure 2. Pooled risk ratio for seroprotection at 5 to 6 months after a 2-dose primary series of inactivated Vero cell culture-derived Japanese encephalitis vaccine (JE-VC) in randomized controlled trials*
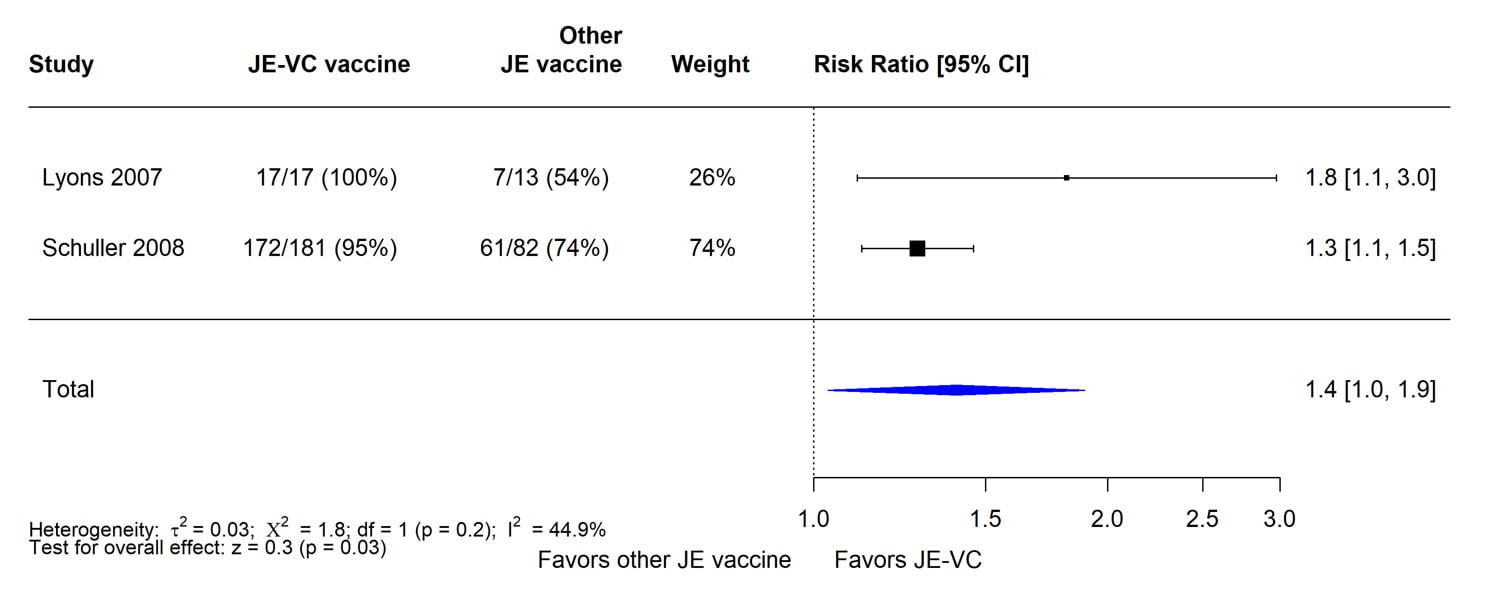
*Pooled risk ratios computed using the random effects model (Mantel-Haenszel method). Risk ratio = Proportion seroprotected in JE-VC group / Proportion seroprotected in other Japanese encephalitis (JE) vaccine group. Risk ratio >1.0 favors JE-VC versus other JE vaccine.
Table 5. Serious adverse events reported within 1 month after either dose of JE-VC
| Study | Sites | Type | Age group | JE-VC | Comparison vaccines[1] | ||
|---|---|---|---|---|---|---|---|
| No. | (%) | No. | (%) | ||||
| Kaltenbock 2010 | India | RCT | Children (1-2 yrs) | 0/48 | (0) | 0/12 | (0) |
| Dubischar 2017(b)[2] | Philippines | RCT | Children (2 mos-17 yrs) | 6/1411 | (<1) | 5/458 | (1) |
| Tauber 2007[3] | U.S./Europe | RCT | Adults (≥18 yrs) | 1/428 | (<1) | 0/435 | (0) |
| Tauber 2008[4] | U.S./Europe/Australia | RCT | Adults (≥18 yrs) | 10/1993 | (1) | 6/657 | (1) |
| Lyons 2007 | U.S. | RCT | Adults (18–49 yrs) | 0/24 | (0) | 0/21 | (0) |
| Biological E 2013 | India | RCT | Adults (18–49 yrs) | 0/54 | (0) | 0/108 | (0) |
| Kaltenbock 2009[5] | Europe | RCT | Adults (≥18 yrs) | 1/127 | (1) | 0/65 | (0) |
| Jelinek 2015(a)[6] | Europe | RCT | Adults (18-65 yrs) | 5/56 | (9) | 1/220 | (<1) |
| Jelinek 2018 | U.S./Europe/Australia | Obs | Adults (≥18 yrs)Children (2 mos–17 yrs) | 1/100 | (0) | — | — |
| Schuller 2009 | Europe | Obs[7] | Adults (≥18 yrs) | 0/125 | (0) | — | — |
| Woolpert 2012 | U.S. | Obs | Adults (≥18 yrs) | 0/123 | (0) | — | — |
| Cramer 2016(a)[8] | Europe | Obs | Adults (64–83 yrs) | 5/200 | (3) | — | — |
Table 5 Footnotes
- Inactivated mouse brain-derived JE vaccine (JenceVac) manufactured by Green Cross (Korea) [Kaltenbock 2010], 7-valent pneumococcal conjugate vaccine (Prevnar) manufactured by Wyeth (now Pfizer Inc.) or hepatitis A vaccine (Havrix 720) manufactured by GSK [Dubischar 2017b], hepatitis A vaccine (Havrix 1440) manufactured by GSK [Kaltenbock 2009], inactivated mouse brain-derived JE vaccine (JE-VAX) manufactured by Biken (Japan) [Tauber 2007; Lyons 2007], phosphate buffered saline with 0.1% aluminum hydroxide [Tauber 2008], inactivated Vero cell culture-derived JE vaccine (JEEV) manufactured by Biological E (India) [Biological E 2013], or purified chick embryo cell culture rabies vaccine (Rabipur)[Jelinek 2015(a); data presented in Jelinek 2015(b)].
- Six serious adverse events following JE-VC included two febrile seizures (2 days after dose 2 and 20 days after dose 1), cellulitis (9 days after dose 2), gastroenteritis and hematoma (12 days after dose 1), pneumonia (23 days after dose 2), and dengue (24 days after dose 1). Five serious adverse events following comparison vaccines included three febrile seizures (9 days and 4 weeks after Havrix and 4 weeks after Prevnar), dyspnea (14 days after Havrix), and gastroenteritis (20 days after Havrix).
- Only serious adverse event following JE-VC was a myocardial infarction at 3 weeks after dose 2.
- Ten serious adverse events following JE-VC included one each of rectal hemorrhage, chest pain, limb abscess, appendicitis, facial injury, facial fracture, ulna fracture, adnexal pain, ovarian cyst, and dermatomyositis. Six serious adverse events after placebo included appendicitis (n=2), acute coronary syndrome, proctalgia, urinary calculus, and circulatory collapse.
- Only serious adverse event following JE-VC was a seizure in a patient with a history of epilepsy.
- Only one event, considered related to vaccination, described and was eyelid edema and generalized pruritus a few hours after first JE-VC dose.
- RCT with no comparative safety data.
- Adverse events collected until day 42 after dose 2 of JE-VC; the five serious adverse events were not described.
Table 6. Serious adverse events reported within 6 to 7 months after the first dose of JE-VC[1]
| Study | Sites | Type | Age group | JE-VC | Comparison vaccines[2] | ||
|---|---|---|---|---|---|---|---|
| No. | (%) | No. | (%) | ||||
| Dubischar 2017(b)[3] | Philippines | RCT | Children (2 mos–17 yrs) | 23/1411 | (2) | 11/458 | (2) |
| Dubischar-Kastner 2010(b)[4] | U.S./Europe/Australia | RCT | Adults (≥18 yrs) | 38/3558 | (1) | 16/1092 | (1) |
| Jelinek 2018[5] | U.S./Europe/Australia | Obs | Children (2 mos–17 yrs) | 3/100 | (3) | — | — |
| Cramer 2016(a)[6] | Europe | Obs | Adults (≥64yrs) | 8/200 | (4) | — | — |
Table 6 Footnotes
- For studies in children, follow-up was at 7 months after the first dose of vaccine. For studies in adults, follow-up was at 6 months after the first dose.
- 7-valent pneumococcal conjugate vaccine (Prevnar) manufactured by Wyeth (now Pfizer Inc.), hepatitis A vaccine (Havrix 720) manufactured by GSK [Dubischar 2017b], or inactivated mouse brain-derived JE vaccine (JE-VAX) manufactured by Biken (Japan) or phosphate buffered saline with 0.1% aluminum hydroxide [Dubischar-Kastner 2010 (b)].
- Serious adverse events reported in 23 JE-VC recipients included pneumonia (n=6), febrile seizures (n=5), dengue (n=2), gastroenteritis (n=2), and one each with hematoma, cellulitis, hepatitis A, strabismus, car accident, Kawasaki disease, typhoid, upper respiratory infection, urinary tract infection, stillbirth, meningitis, and disseminated intravascular coagulation. One subject had two preferred terms reported (gastroenteritis and hematoma) and two subjects had three preferred terms reported (one with meningitis, pneumonia and disseminated intravascular coagulation, and one with dengue, pharyngitis, and upper respiratory infection). The stillbirth occurred in a subject who became pregnant >4 months after vaccination. One death occurred in a 12 year old male with meningitis, pneumonia, and disseminated intravascular coagulation with onset 4 months after he received dose 2 of JE-VC. Serious adverse events in 11 comparison vaccine recipients included febrile seizures (n=4), pneumonia (n=3), dengue (n=1), gastroenteritis (n=1), familial periodic paralysis (n=1), hyponatremia (n=1), dyspnea (n=1); one subject had two preferred terms reported.
- The 38 serious adverse events occurring within 6 months following receipt of JE-VC were not delineated. One death occurred in a 70 year old female with adenocarcinoma of the lung diagnosed 1 month after she received dose 2 of JE-VC.
- Three serious adverse events in JE-VC recipients included one subject each with diabetes mellitus (3 months after dose 2), dizziness (4 months after dose 2), and intentional self-injury; only 92 subjects completed the study but timing of discontinuation is unknown.
- The eight serious adverse events were not described.
Table 7. Serious adverse events reported through post-marketing surveillance following receipt of JE-VC[1]
| Study | Countries | Reporting
Period |
Doses
distributed |
Serious adverse events reported | |
|---|---|---|---|---|---|
| No. | Rate per 100,000 doses distributed | ||||
| Schuller 2011[2] | U.S./Europe/Australia | Apr 2009–Mar 2010 | 246,687 | 4[3] | 1.6 |
| Rabe 2015[2] | U.S. | May 2009–Apr 2012 | 275,848 | 5[4] | 1.8 |
| Walker 2018 | U.S. | May 2012–Apr 2016 | 802,229 | 9[5] | 1.1 |
| Butler 2017 | U.S. | Nov 2011–Aug 2014 | 145[6] | 0 | 0 |
Table 7 Footnotes
- Adverse events reported through VAERS and other similar passive surveillance systems may or may not be causally related to the vaccine.
- 85,583 doses distributed in the United States from May 2009–March 2010 are included in both studies, but there was no overlap in the 9 serious adverse events identified in the two studies.
- One report each of neuritis (9 hours after vaccination), oropharyngeal spasm (day of vaccination), meningismus (1 day after vaccination), and iritis (1 day after vaccination). The patient with iritis had also received typhoid vaccine.
- Includes one report each of an immediate hypersensitivity reaction (day of vaccination), a delayed hypersensitivity reaction (2 days after vaccination), appendicitis (5 days after vaccination), myopericarditis (11 days after vaccination), and encephalomyelitis (39 days after vaccination). Two serious adverse events occurred after administration of JE-VC alone and three events occurred after concomitant administration of JE-VC with other vaccines.
- Includes one death that occurred 8 days after JE-VC administration as a result of cardiovascular collapse due to ischemic heart disease. The remaining eight events included cardiomyopathy (n=1), myocardial infarct/acute myocarditis (n=1), angina pectoris (n=1), systemic febrile reaction (n=1), acute kidney injury/myopathy (n=1), anaphylaxis (n=1), immediate hypersensitivity (15 minutes after administration of JE-VC) (n=1), and seizure (n=1). Eight of the serious events occurred following administration of JE-VC concomitantly with other vaccines.
- Doses administered
Figure 3. Pooled risk ratio for serious adverse events within 1 month after either dose of inactivated Vero cell culture-derived Japanese encephalitis vaccine (JE-VC) in randomized controlled trials*
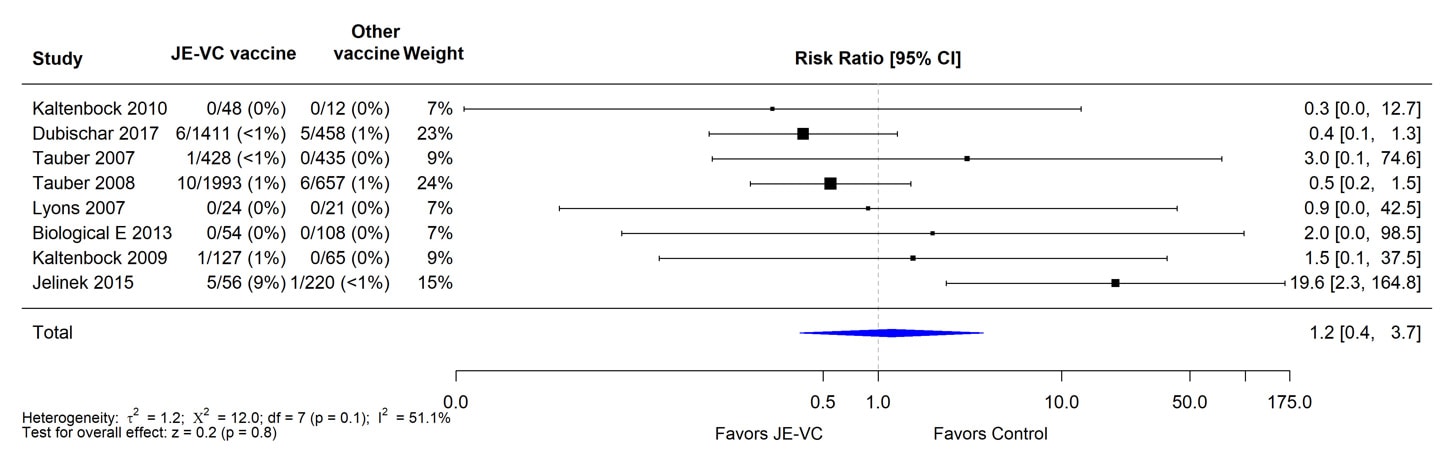
*Pooled risk ratios computed using the random effects model (Mantel-Haenszel method). Risk ratio = Proportion with serious adverse event in JE-VC group / Proportion with serious adverse event in control vaccine group. Risk ratio <1.0 favors JE-VC versus control vaccine.
Figure 4. Pooled risk ratio for serious adverse events within 6 to 7 months after the first dose of inactivated Vero cell culture-derived Japanese encephalitis vaccine (JE-VC) in randomized controlled trials*
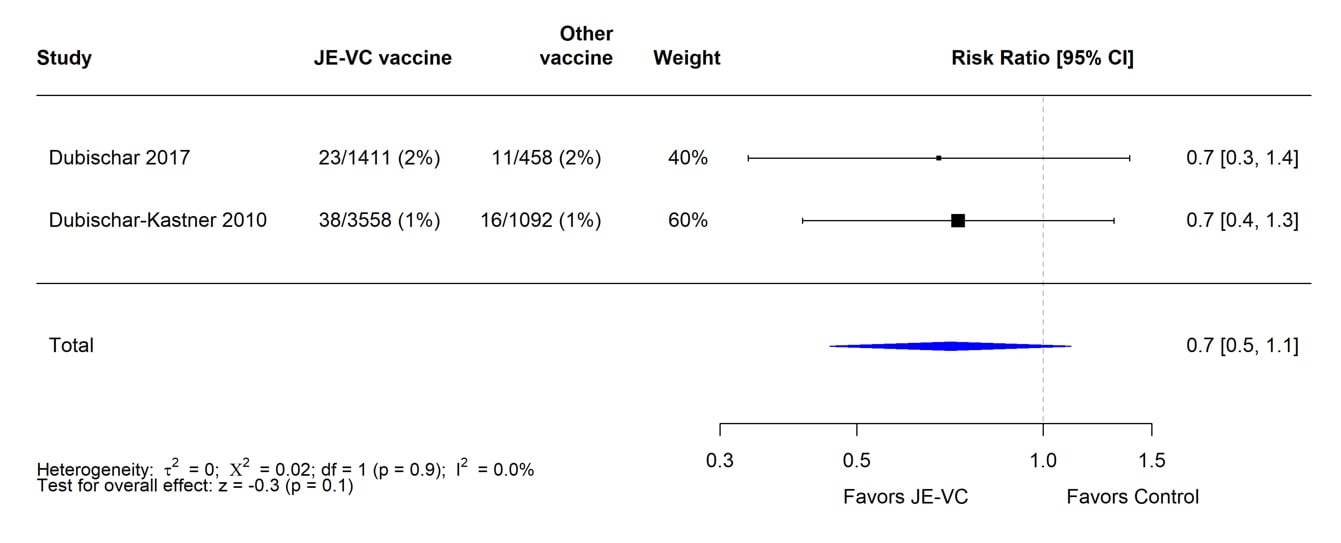
*Pooled risk ratios computed using the random effects model (Mantel-Haenszel method). Risk ratio = Proportion with serious adverse event in JE-VC group / Proportion with serious adverse event in control vaccine group. Risk ratio <1.0 favors JE-VC versus control vaccine.
Table 8. Fever reported as a solicited adverse event within 7 days after either dose of JE-VC
| Study | Sites | Type | Age group | JE-VC | Comparison vaccines[1] | ||
|---|---|---|---|---|---|---|---|
| No. | (%) | No. | (%) | ||||
| Kaltenbock 2010[2] | India | RCT | Children (1–2 yrs) | 1/48 | (2) | 1/12 | (8) |
| Dubischar 2017(b)[3] | Philippines | RCT | Children (2 mos–17 yrs) | 216/1411 | (15) | 62/458 | (14) |
| Tauber 2008[4] | U.S./Europe/Australia | RCT | Adults (≥18 yrs) | 64/1993 | (3) | 20/657 | (3) |
| Lyons 2007[5] | U.S. | RCT | Adults (18–49 yrs) | 5/24 | (21) | 1/21 | (5) |
| Jelinek 2018[3] | U.S./Europe/Australia | Obs | Children (2 mos–17 yrs) | 4/100 | (4) | — | — |
| Woolpert 2012[6] | U.S. | Obs | Adults (≥18 yrs) | 6/116 | (5) | — | — |
| Cramer 2016(a) | Europe | Obs | Adults (≥64 yrs) | 0/200 | (0) | — | — |
Table 8 Footnotes
- Inactivated mouse brain-derived JE vaccine (JenceVac) manufactured by Green Cross (Korea) [Kaltenbock 2010], 7-valent pneumococcal conjugate vaccine (Prevnar) manufactured by Wyeth (now Pfizer Inc.) or hepatitis A vaccine (Havrix) manufactured by GSK [Dubischar 2017(b)], phosphate buffered saline with 0.1% aluminum hydroxide [Tauber 2008], or inactivated mouse brain-derived JE vaccine (JE-VAX) manufactured by Biken (Japan) [Lyons 2007].
- Fever defined as temperature ≥38.0C.
- Exact numbers not provided so calculated from percentages; fever defined as ≥37.7C.
- May include unsolicited adverse events of fever that occurred up to 28 days after either dose of JE-VC
- Fever defined as ≥37.6C.
- Fever was collected for 4 days following vaccination and was subjective as reported by the participant.
Table 9. Rash reported as a solicited adverse event within 7 days after either dose of JE-VC
| Study | Sites | Type | Age group | JE-VC | Comparison vaccines[1] | ||
|---|---|---|---|---|---|---|---|
| No. | (%) | No. | (%) | ||||
| Kaltenbock 2010 | India | RCT | Children (1–2 yrs) | 1/48 | (2) | 0/12 | (0) |
| Dubischar 2017(b) | Philippines | RCT | Children (2 mos–17 yrs) | 48/1411 | (3) | 15/458 | (3) |
| Tauber 2008[2] | U.S./Europe/Australia | RCT | Adults (≥18 yrs) | 26/1993 | (1) | 10/657 | (2) |
| Lyons 2007 | U.S. | RCT | Adults (18–49 yrs) | 0/24 | (0) | 1/21 | (5) |
| Jelinek 2018 | U.S./Europe/Australia | Obs | Children (2 mos–17 yrs) | 4/100 | (4) | — | — |
| Woolpert 2012[3] | U.S. | Obs | Adults (≥18 yrs) | 2/116 | (2) | — | — |
| Cramer 2016(a) | Europe | Obs | Adults (≥64 yrs) | 0/200 | (0) | — | — |
Table 9 Footnotes
- Inactivated mouse brain-derived JE vaccine (JenceVac) manufactured by Green Cross [Kaltenbock 2010], 7-valent pneumococcal conjugate vaccine (Prevnar) manufactured by Wyeth (now Pfizer Inc.) or hepatitis A vaccine (Havrix) manufactured by GSK [Dubischar 2017b], phosphate buffered saline with 0.1% aluminum hydroxide [Tauber 2008], or inactivated mouse brain-derived JE vaccine (JE-VAX) manufactured by Biken (Japan) [Lyons 2007].
- May include unsolicited adverse events of rash that occurred up to 28 days after either dose of JE-VC.
- Rash was collected for 4 days following vaccination
Table 10. Hypersensitivity or urticaria[1] reported as an unsolicited adverse event within 1 month after either dose of JE-VC
| Study | Sites | Type | Age group | JE-VC | Comparison vaccines[2] | ||
|---|---|---|---|---|---|---|---|
| No. | (%) | No. | (%) | ||||
| Kaltenbock 2010 | India | RCT | Children (1–2 yrs) | 0/48 | (0) | 0/12 | (0) |
| Dubischar 2017(b)[3] | Philippines | RCT | Children (2 mos–17 yrs) | 4/1411 | (<1) | 0/458 | (0) |
| Tauber 2008 | U.S./Europe/Australia | RCT | Adults (=18 yrs) | 1/1993 | (<1) | 1/657 | (<1) |
| Jelinek 2018 | U.S./Europe/Australia | Obs | Children (2 mos–17 yrs) | 5/100 | (5) | — | — |
| Woolpert 2012 | U.S. | Obs | Adults (=18 yrs) | 0/116 | (0) | — | — |
| Cramer 2016(a)[4] | Europe | Obs | Adults (=64 yrs) | 5/200 | (3) | — | — |
Table 10 Footnotes
- Unsolicited adverse events reported within 28 days after vaccination and classified by the study investigator as hypersensitivity or urticaria.
- Inactivated mouse brain-derived JE vaccine (JenceVac) manufactured by Green Cross [Kaltenbock 2010], 7-valent pneumococcal conjugate vaccine (Prevnar) manufactured by Wyeth (now Pfizer Inc.) or hepatitis A vaccine (Havrix) manufactured by GSK [Dubischar 2017(b)], or phosphate buffered saline with 0.1% aluminum hydroxide [Tauber 2008].
- Events within 14 days of either dose of JE-VC
- Adverse events collected until day 42 after dose 2 of JE-VC.
Table 11. Neurologic adverse events[1] reported as an unsolicited adverse event within 1 month after either dose of JE-VC
| Study | Sites | Type | Age group | JE-VC | Comparison vaccines[2] | ||
|---|---|---|---|---|---|---|---|
| No. | (%) | No. | (%) | ||||
| Kaltenbock 2010 | India | RCT | Children (1–2 yrs) | 0/48 | (0) | 0/12 | (0) |
| Dubischar 2017(b) | Philippines | RCT | Children (2 mos–17 yrs) | 0/1411 | (0) | 0/458 | (0) |
| Tauber 2008 | U.S./Europe/Australia | RCT | Adults (=18 yrs) | 26/1993 | (1) | 8/657 | (1) |
| Jelinek 2018 | U.S./Europe/Australia | Obs | Children (2 mos–17 yrs) | 0/100 | (0) | — | — |
| Woolpert 2012 | U.S. | Obs | Adults (=18 yrs) | 0/116 | (0) | — | — |
Table 11 Footnotes
- Unsolicited adverse events reported within 28 days after vaccination and classified by the study investigator as nervous system disorder other than headaches. No cases of meningitis, encephalitis, acute disseminated encephalomyelitis, or Guillain Barré syndrome were reported among JE-VC or comparison vaccine recipients.
- Inactivated mouse brain-derived JE vaccine (JenceVac) manufactured by Green Cross [Kaltenbock 2010], 7-valent pneumococcal conjugate vaccine (Prevnar) manufactured by Wyeth (now Pfizer, Inc.) or hepatitis A vaccine (Havrix) manufactured by GSK [Dubischar 2017b], or phosphate buffered saline with 0.1% aluminum hydroxide [Tauber 2008].
Table 12. Medically attended adverse events within 1 month after either dose of JE-VC
| Study | Sites | Type | Age group | JE-VC | Comparison vaccines[1] | ||
|---|---|---|---|---|---|---|---|
| No. | (%) | No. | (%) | ||||
| Dubischar 2017(b)[2] | Philippines | RCT | Children (2 mos–17 yrs) | 256/1411 | (18) | 83/458 | (18) |
| Tauber 2008 | U.S./Europe/Australia | RCT | Adults (=18 yrs) | 254/1993 | (13) | 80/657 | (12) |
| Kaltenbock 2009 | Europe | RCT | Adults (=18 yrs) | 11/127 | (9) | 11/65 | (17) |
| Jelinek 2018[3] | U.S./Europe/Australia | Obs | Children (2 mos–17 yrs) | 12/100 | (12) | — | — |
| Woolpert 2012 | U.S. | Obs | Adults (=18 yrs) | 0/116 | (0) | — | — |
| Cramer 2016(a)[4] | Europe | Obs | Adults (=64 yrs) | 38/200 | (19) | — | — |
Table 12 Footnotes
- 7-valent pneumococcal conjugate vaccine (Prevnar) manufactured by Wyeth (now Pfizer Inc.) or hepatitis A vaccine (Havrix 720) manufactured by GlaxoSmithKline [Dubischar 2017(b)], phosphate buffered saline with 0.1% aluminum hydroxide [Tauber 2008], or hepatitis A vaccine (Havrix 1440) manufactured by GSK [Kaltenbock 2009].
- Majority of medically attended events in both groups were respiratory, gastrointestinal, or skin infections.
- Medically attended events affecting at least 2 subjects included nasopharyngitis, bronchitis, diarrhea, hematochezia, nausea, upper abdominal pain, vomiting, pyrexia, and cough.
- Adverse events collected until day 42 after dose 2 of JE-VC.
Table 13. Hypersensitivity reactions reported through post-marketing surveillance following receipt of JE-VC[1]
| Study | Countries | Reporting Period | Doses distributed | Hypersensitivity reactions | |
|---|---|---|---|---|---|
| No. | Rate per 100,000 doses distributed | ||||
| Schuller 2011[2] | U.S./Europe/Australia | Apr 2009–Mar 2010 | 246,687 | 10[3] | 4.1 |
| Rabe 2015[2] | U.S. | May 2009–Apr 2012 | 275,848 | 12[4] | 4.4 |
| Walker 2018 | U.S. | May 2012–Apr 2016 | 802,229 | 24[5] | 3.0 |
| Taucher 2017 | U.S | Jul 2010-May 2011 | 36,358[6] | 9[7] | 24.8 |
Table 13 Footnotes
- Adverse events reported through VAERS and other similar passive surveillance systems may or may not be causally related to the vaccine.
- 85,583 doses distributed in the United States from May 2009–March 2010 are included in both studies
- Includes five reports of rash, and one report each of urticaria, glossodynia, oral hypoaesthesia/swollen tongue, oropharyngeal spasm, pruritus. One (oropharyngeal spasm) was classified as a serious adverse event.
- The 12 events included occurred within 10 days after vaccination. Seven hypersensitivity reactions occurred after administration of JE-VC alone and five events occurred after concomitant administration of JE-VC with other vaccines. Two reports were classified as serious adverse events.
- The 24 events included one report of anaphylaxis, seven immediate hypersensitivity events (occurred <2 hours after administration of JE-VC), 15 delayed hypersensitivity events, and one hypersensitivity event that occurred within 1 day after administration but lacked more specific timing data to classify as immediate or delayed hypersensitivity. Two reports were classified as serious adverse events. Fifteen (63%) events, including both serious events, occurred after concomitant administration of JE-VC with other vaccines.
- Doses administered
- Includes four reports of events with ICD-9 codes for delayed hypersensitivity/serum sickness, two reports with codes for anaphylactic shock, and three reports with codes for angioedema. For four reports there was concurrent administration of other vaccines.
Table 14. Neurologic adverse events other than headaches reported through post-marketing surveillance following receipt of JE-VC[1]
| Study | Countries | Reporting Period | Doses distributed | Neurologic adverse events | |
|---|---|---|---|---|---|
| No. | Rate per 100,000 doses distributed | ||||
| Schuller 2011[2] | U.S./Europe/Australia | Apr 2009–Mar 2010 | 246,687 | 2[3] | (0) |
| Rabe 2015[2] | U.S. | May 2009–Apr 2012 | 275,848 | 3[4] | (<1) |
| Walker 2018 | U.S. | May 2012–Apr 2016 | 802,229 | 2[5] | (0) |
| Taucher 2017 | U.S. | Jul 2010-May 2011 | 36,358[6] | 8[7] | (1) |
Table 14 Footnotes
- Adverse events reported through VAERS and other similar passive surveillance systems may or may not be causally related to the vaccine.
- 85,583 doses distributed in the United States from May 2009–March 2010 are included in both studies
- Includes one report each of neuritis and meningism.
- Includes one report of encephalomyelitis at 39 days after vaccination with JE-VC and two reports of seizures on the day of vaccination. All subjects had received other vaccines. One of the reports was classified as a serious adverse event.
- Two reports of seizures, both following concomitant administration of JE-VC with other vaccines. One classified as serious.
- Doses administered
- Includes 6 reports with codes for convulsions and 2 with a code for meningitis. For four reports there was concurrent administration of other vaccines.
Figure 5. Pooled risk ratio for fever as a solicited adverse event within 7 days after either dose of inactivated Vero cell culture-derived Japanese encephalitis vaccine (JE-VC) in randomized controlled trials*
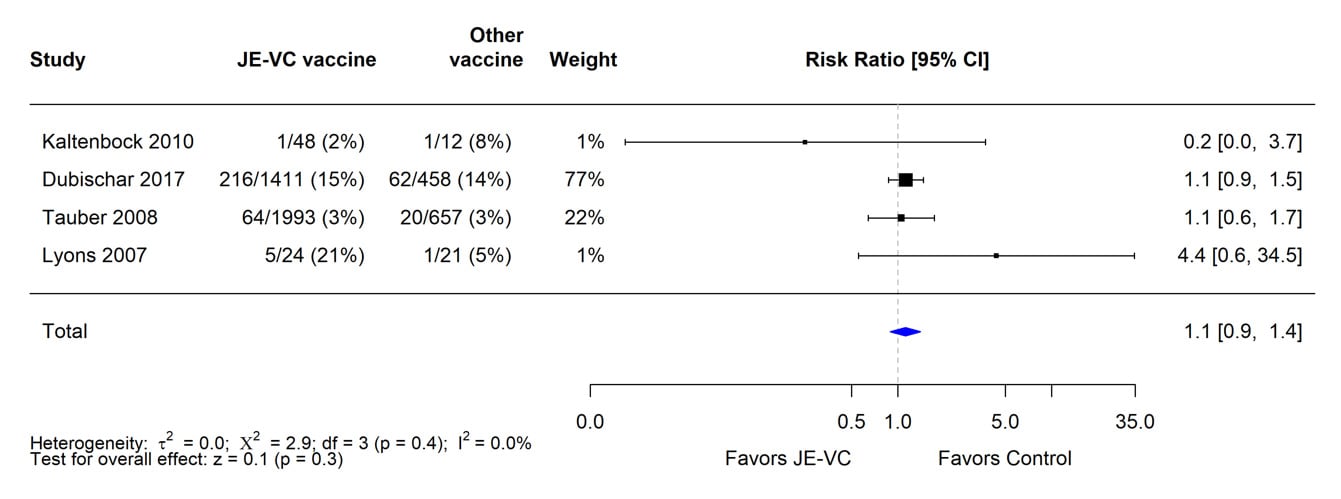
*Pooled risk ratios computed using the random effects model (Mantel-Haenszel method). Risk ratio = Proportion with fever in JE-VC group / Proportion with fever in control vaccine group. Risk ratio <1.0 favors JE-VC versus control vaccine.
Figure 6. Pooled risk ratio for rash as a solicited adverse event within 7 days after either dose of inactivated Vero cell culture-derived Japanese encephalitis vaccine (JE-VC) in randomized controlled trials*
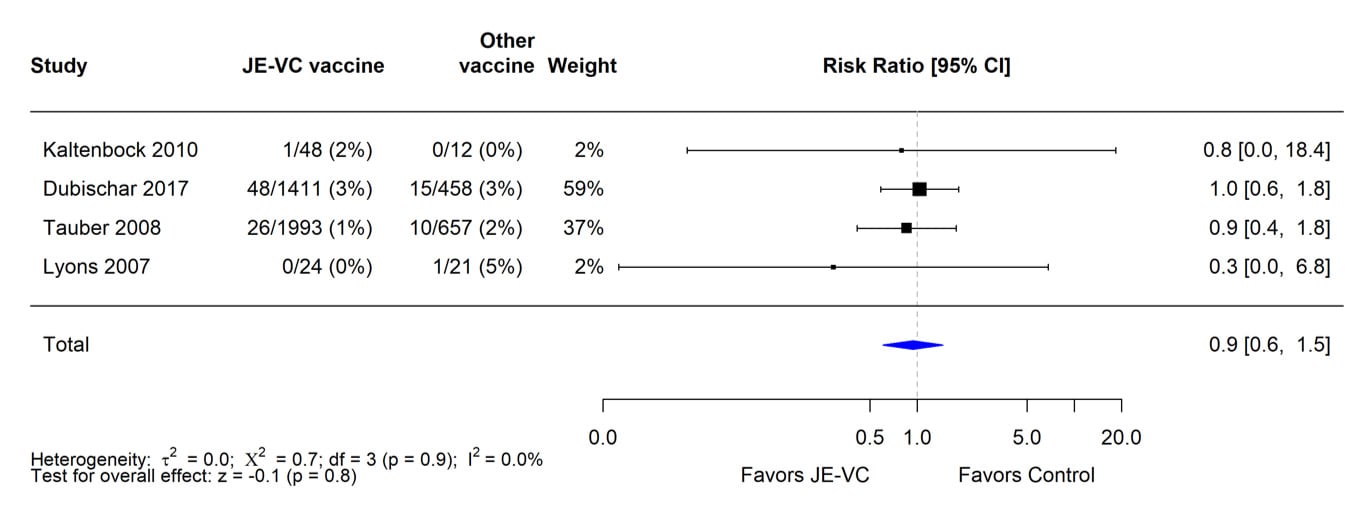
*Pooled risk ratios computed using the random effects model (Mantel-Haenszel method). Risk ratio = Proportion with rash in JE-VC group / Proportion with rash in control vaccine group. Risk ratio <1.0 favors JE-VC versus control vaccine.
Figure 7. Pooled risk ratio for hypersensitivity or urticaria as an unsolicited adverse event within 1 month after either dose of inactivated Vero cell culture-derived Japanese encephalitis vaccine (JE-VC) in randomized controlled trials*
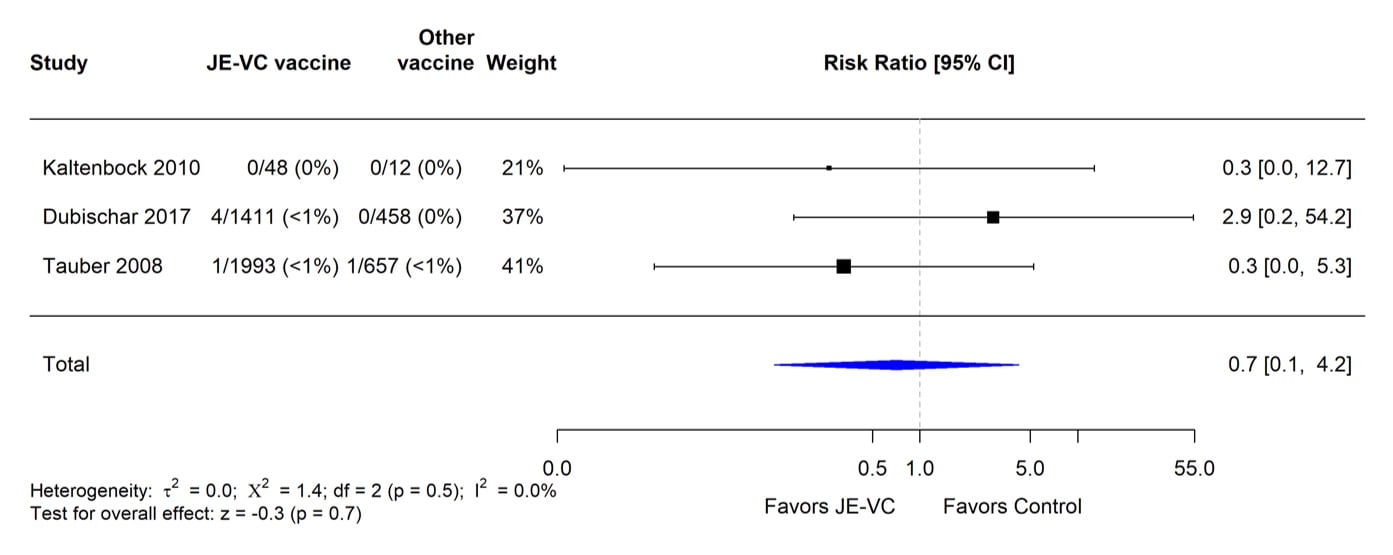
*Pooled risk ratios computed using the random effects model (Mantel-Haenszel method). Risk ratio = Proportion with hypersensitivity or urticaria in JE-VC group / Proportion with hypersensitivity or urticaria in control vaccine group. Risk ratio <1.0 favors JE-VC versus control vaccine.
Figure 8. Pooled risk ratio for neurologic adverse events other than headache within 1 month after either dose of inactivated Vero cell culture-derived Japanese encephalitis vaccine (JE-VC) in randomized controlled trials*
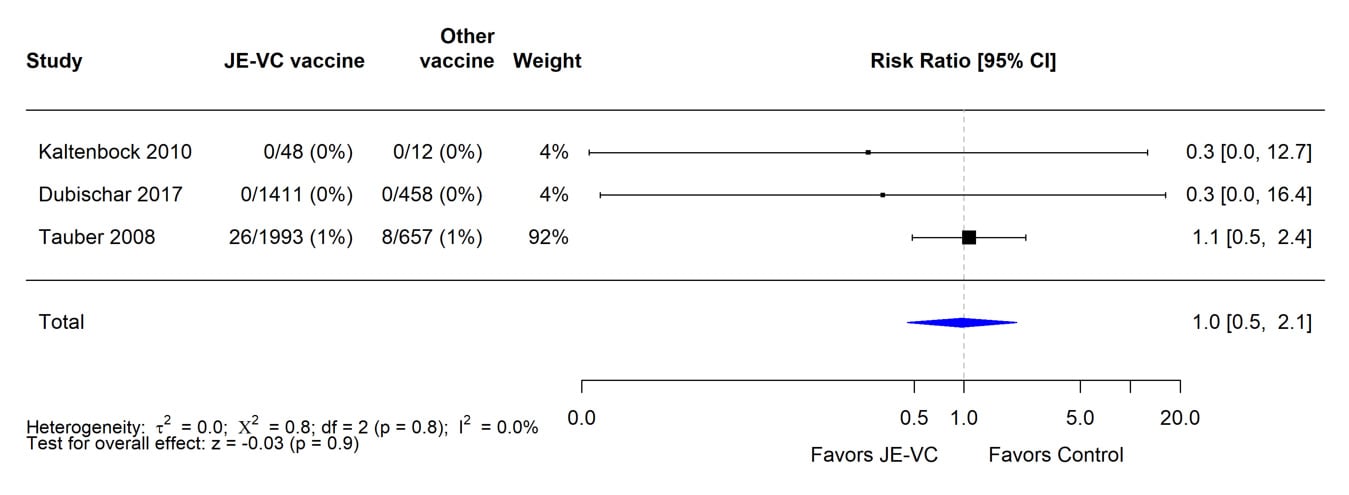
*Pooled risk ratios computed using the random effects model (Mantel-Haenszel method). Risk ratio = Proportion with neurologic adverse events in JE-VC group / Proportion with neurologic adverse events in control vaccine group. Risk ratio <1.0 favors JE-VC versus control vaccine.
Figure 9. Pooled risk ratio for medically attended adverse events within 1 month after either dose of inactivated Vero cell culture-derived Japanese encephalitis vaccine (JE-VC) in randomized controlled trials*
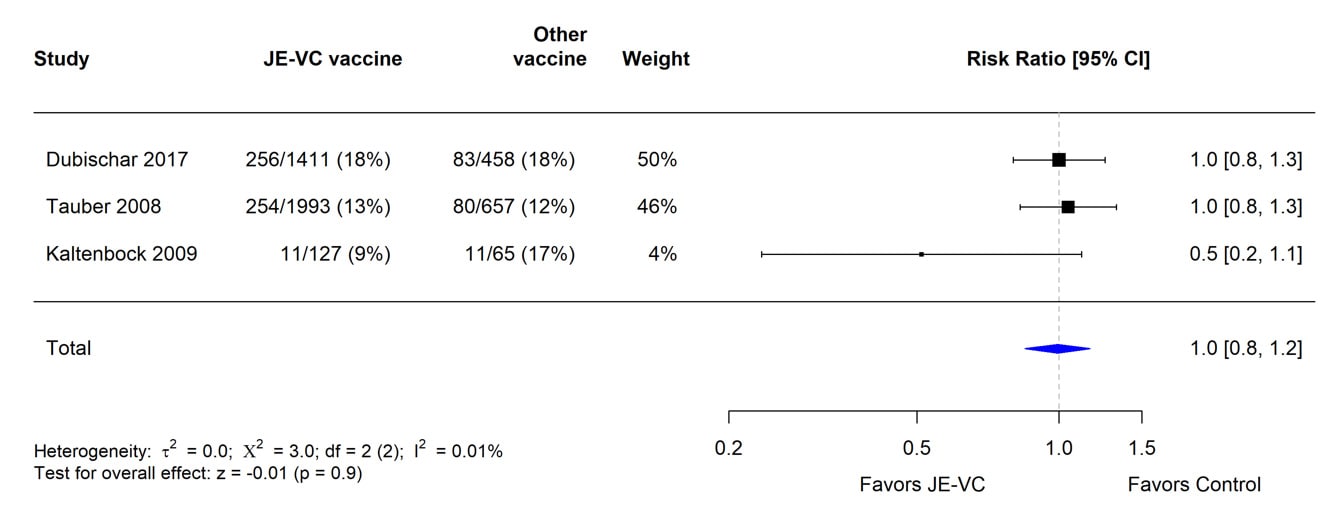
*Pooled risk ratios computed using the random effects model (Mantel-Haenszel method). Risk ratio = Proportion with medically attended adverse events in JE-VC group / Proportion with medically attended adverse events in control vaccine group. Risk ratio <1.0 favors JE-VC versus control vaccine.
Table 15. Limitations and evidence type for benefits and harms for JE-VC
| Outcome | Design(# studies) | Risk of bias | Inconsistency | Indirectness | Imprecision | Other[1] | Evidence type[2] |
|---|---|---|---|---|---|---|---|
| Benefits | |||||||
| Seroprotection at 1 month | RCT (4) | No serious | No serious | No serious | No serious | None | 1 |
| Obs (8) | No serious | No serious | No serious | No serious | None | 3 | |
| Seroprotection at 6 months | RCT (2) | No serious | No serious | No serious | No serious | None | 1 |
| Obs (4) | No serious | No serious | No serious | No serious | None | 3 | |
| Harms | |||||||
| Serious adverse events | RCT (8) | Yes[3] | No serious | No serious | No serious | None | 2 |
| Obs (8) | No serious | No serious | No serious | No serious | None | 3 | |
| Adverse events of special interest | RCT (5) | Yes[3] | No serious | No serious | No serious | None | 2 |
| Obs (7) | No serious | No serious | No serious | No serious | None | 3 | |
Table 15 Footnotes
RCT = Randomized controlled trial with JE-VC; Obs = Observational study, RCT without comparative data for the outcome measure, or post-marketing surveillance data.
- Publication bias, strength of association, dose response, or opposing plausible residual confounding.
- Evidence type:
- RCTs or overwhelming evidence from observational studies
- RCTs with important limitations, or exceptionally strong evidence from observational studies
- Observational studies, or RCTs with notable limitations
- Clinical experience and observations, observational studies with important limitations, or RCTs with several major limitations
- Risk of bias due to inadequate blinding of study participants and personnel.
Table 16. Overall quality of evidence for JE-VC
| Outcome | Study design (# studies) | Finding | Evidence type 1, 2 | Overall quality of evidence |
|---|---|---|---|---|
| Seroprotection at 1 month | RCT (4) | High (≥95%) at 1 month in all but one study | 1 | 1 |
| Seroprotectionat 6 months | RCT (2) | Maintained (83-100%) at 6 months | 1 | |
| Serious adverse events | RCT (8) | Low incidence; similar to comparison vaccines | 2 | 2 |
| Adverse events of special interest | RCT (5) | Similar to comparison vaccines | 2 |
Table 16 Footnotes
RCT = Randomized controlled trial.
- Evidence Type:
- RCTs or overwhelming evidence from observational studies
- RCTs with important limitations, or exceptionally strong evidence from observational studies
- Observational studies, or RCTs with notable limitations
- Clinical experience and observations, observational studies with important limitations, or RCTs with several major limitations
- Body of evidence for each outcome includes both RCTs and observational studies; the study design that provides higher quality of evidence was selected.