Model Performance Evaluation Program Report of Results: September 2020
MPEP September 2020 pdf icon[PDF – 1 MB]
The purpose of this report is to present results of the U.S. Centers for Disease Control and Prevention (CDC) Model Performance Evaluation Program (MPEP) for Mycobacterium tuberculosis complex (MTBC) drug susceptibility testing survey sent to participants in September 2020.
Primary Classification
This report contains DST results submitted to CDC by survey participants at 69 laboratories in 34 states.
The participants were asked to indicate the primary classification of their laboratory (Figure 1). MPEP participants self-classified as:
- 50 (73%): Public health laboratory (e.g., local, county, state)
- 9 (13%): Hospital laboratory
- 7 (10%): Independent/Reference laboratory (non-hospital based)
- 2 (3%): Federal government laboratory
- 1 (1%): Other (Medical Manufacturing Company)
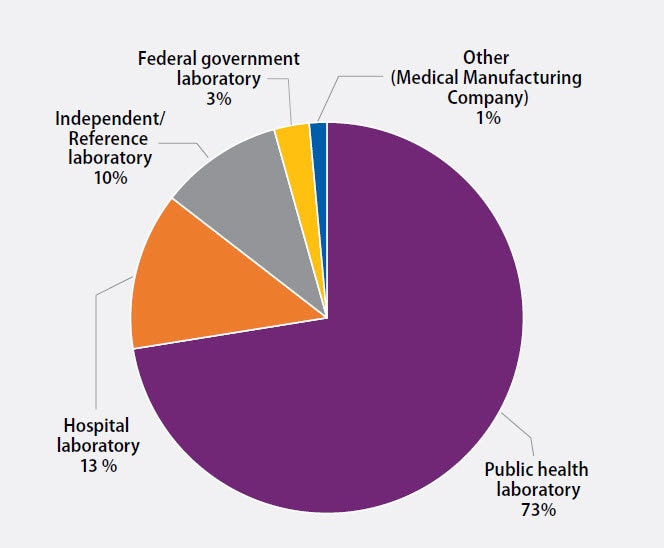
Figure 1. Primary Classification of Participating Laboratories, September 2020
Annual Number of MTBC Drug Susceptibility Tests Performed
The number of MTBC isolates tested for drug susceptibility by the 69 participants in 2019 (excluding isolates used for quality control) is shown in Figure 2. In 2019, the counts ranged from 0 to 1,039 tests. Participants at 28 (41%) laboratories reported testing 50 or fewer DST isolates per year. Laboratories with low MTBC DST volumes are encouraged to consider referral of testing because of concerns about maintaining proficiency [7].
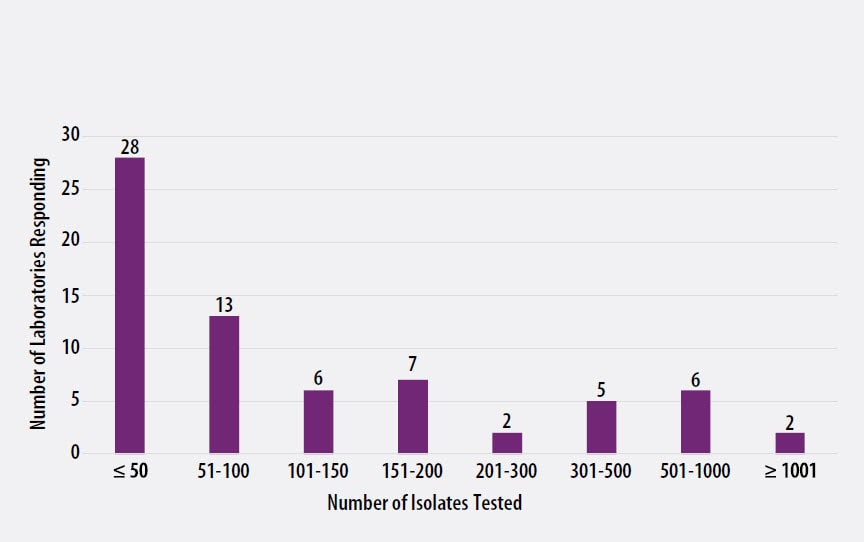
Figure 2. Distribution of the Annual Volume of MTBC Isolates Tested for Drug Susceptibility by Participants in Previous Calendar Year (n=69)
MTBC DST Methods Used by Participants
The DST methods that were used by participating laboratories for this panel of MTBC isolates are displayed in Figure 3. Of participating laboratories, 46 (66%) reported results for only one method, 20 (29%) reported two methods, and 4 (5%) noted three susceptibility methods
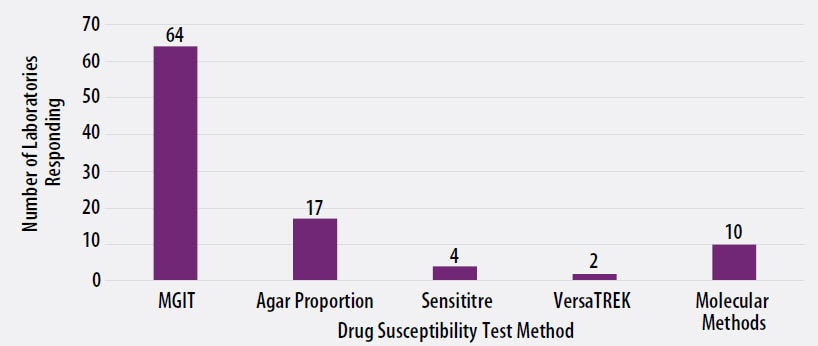
Figure 3. MTBC Drug Susceptibility Test Method Used by Participants (n=97)
Ten molecular methods reported by participants are shown in Figure 4. The method used most frequently by laboratories (5) was targeted DNA sequencing (50%), including pyrosequencing and Sanger sequencing. Three (30%) laboratories reported use of the Cepheid Xpert MTB/RIF assay, one (10%) reported results for line probe assays, Genotype MTBDRplus and MTBDRsl by Bruker, and one (10%) reported results from whole genome sequencing.
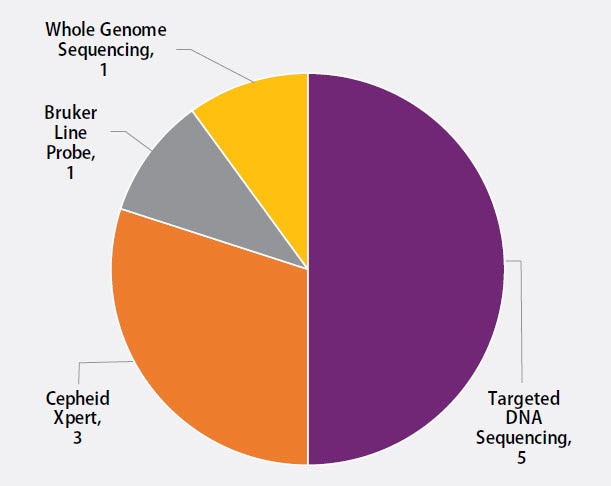
Figure 4. Molecular Method Reported (n=10)
Antituberculosis Drugs Tested by Participants
The number of participating laboratories that reported testing each antituberculosis drug in the September 2020 survey is presented in Figure 5. CLSI recommends testing a full panel of first-line drugs (rifampin [RMP], isoniazid [INH], ethambutol [EMB] and pyrazinamide [PZA])[1] because it represents a combination of tests that provides the clinician with comprehensive information related to the four-drug antituberculosis therapy currently recommended for most patients. All participants reported results for three of the first-line drugs (RMP, INH and EMB) and 64 (93%) also reported results for PZA by growth-based DST methods. One laboratory performs molecular testing for PZA via sequencing of pncA, in place of growth-based DST.
For 21 laboratories reporting second-line drug results (with the exception of streptomycin), four (19%) tested all three second-line injectable drugs and at least one fluoroquinolone needed to confidently define XDR TB. The second-line injectable drugs are amikacin, kanamycin and capreomycin. Fluoroquinolones include ofloxacin, ciprofloxacin, levofloxacin and moxifloxacin.
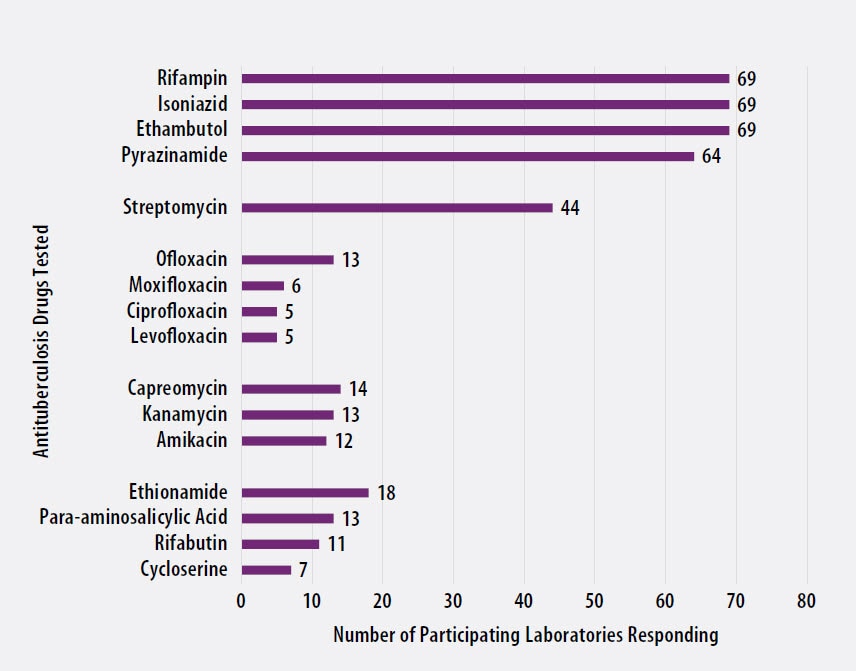
Figure 5. Antituberculosis Drugs Tested by Participants
Anticipated growth-based and molecular results for the panel of MTBC isolates sent to participants in September 2020 are shown in the tables below. Although CDC recommends broth-based methods for routine first-line DST of MTBC isolates, the results obtained by the reference agar proportion method (except for pyrazinamide, in which MGIT was performed) are shown in Table 1. Molecular results obtained by DNA sequencing are listed in Table 2 [4].
Table 1. Expected Growth-based Results for September 2020 Survey
Note—S=susceptible, R=resistant, V=variable
| Isolate | RMP | INH | EMB | PZA | Second-line Drugs Resistant to: |
| 2020F* | S | R† | S | S | ETA |
| 2020G | S | R | S | S | ETA |
| 2020H | S | R | R | S | ETA, STR¥ |
| 2020I* | S | R† | S | S | ETA |
| 2020J | R | S | S | S |
* Isolates 2020F and 2020I are the same isolate
†Although INH resistance was expected, 80% consensus for a single categorical result of either susceptible or resistant was not achieved for this isolate among participating laboratories. Variable resistance was observed depending on growth-based DST method.
¥Although STR resistance was expected, 80% consensus for a single categorical result of either susceptible or resistant was not achieved for this isolate among participating laboratories. Variable resistance was observed depending on growth-based DST method.
Table 2. Expected Molecular Results (Mutations Detected in Loci Associated with Resistance) for September 2020 Survey
Note—Empty cell=No mutation detected
| Isolate | rpoB¥ | katG | inhA | fabG1 | embB | ethA |
| 2020F | Arg447Arg*
(Arg528Arg)† |
Leu203Leu | ||||
| 2020G | C-15T | |||||
| 2020H | Ser315Thr | Met306Val | (partial deletion) | |||
| 2020I | Arg447Arg*
(Arg528Arg)† |
Leu203Leu | ||||
| 2020J | Ser450Leu*
(Ser531Leu)† |
¥Mutation is listed using both the M. tuberculosis and E.coli numbering system [5, 6]
* M. tuberculosis numbering system used
†E. coli numbering system used
- CLSI, Susceptibility Testing of Mycobacteria, Nocardiae spp., and Other Aerobic Actinomycetes, in 3rd Ed. CLSI Standard M24. 2018, Clinical and Laboratory Standards Institute: Wayne, PA.
- World Health Organization, Technical Report on critical concentrations for drug susceptibility testing of isoniazid and the rifamycins (rifampicin, rifabutin and rifapentine). 2021: Geneva.
- World Health Organization, Technical Report on critical concentrations for drug susceptibility testing of medicines used in the treatment of drug-resistant tuberculosis. 2018: Geneva.
- Campbell, P.J., et al., Molecular detection of mutations associated with first- and second-line drug resistance compared with conventional drug susceptibility testing of Mycobacterium tuberculosis. Antimicrob Agents Chemother, 2011. 55(5): p. 2032-41.
- Andre, E., et al., Consensus numbering system for the rifampicin resistance-associated rpoB gene mutations in pathogenic mycobacteria. Clin Microbiol Infect, 2017. 23(3): p. 167-172.
- APHL, Issues in Mycobacterium tuberculosis complex (MTBC) Drug Susceptibility Testing: Rifampin (RIF), in APHL Issues in Brief: Infectious Diseases. 2019, Association of Public Health Laboratories: Washington, D.C.
- APHL, TB Drug Susceptibility Testing Expert Panel Meeting Summary Report. 2007, Association of Public Health Laboratories: Washington, D.C.
- Almeida Da Silva, P.E. and J.C. Palomino, Molecular basis and mechanisms of drug resistance in Mycobacterium tuberculosis: classical and new drugs. J Antimicrob Chemother, 2011. 66(7): p. 1417-30.
- Zhang, Y. and W.W. Yew, Mechanisms of drug resistance in Mycobacterium tuberculosis. Int J Tuberc Lung Dis, 2009. 13(11): p. 1320-30.
- Ramaswamy, S.V., et al., Single nucleotide polymorphisms in genes associated with isoniazid resistance in Mycobacterium tuberculosis. Antimicrob Agents Chemother, 2003. 47(4): p. 1241-50.
- Ando, H., et al., A silent mutation in mabA confers isoniazid resistance on Mycobacterium tuberculosis. Mol Microbiol, 2014. 91(3): p. 538-47.
- Morlock, G.P., et al., ethA, inhA, and katG loci of ethionamide-resistant clinical Mycobacterium tuberculosis isolates. Antimicrob Agents Chemother, 2003. 47(12): p. 3799-805.
- Centers for Disease Control and Prevention, Availability of an assay for detecting Mycobacterium tuberculosis, including rifampin-resistant strains, and considerations for its use – United States, 2013. MMWR Morb Mortal Wkly Rep, 2013. 62(41): p. 821-7.
- Van Deun, A., et al., Rifampin drug resistance tests for tuberculosis: challenging the gold standard. J Clin Microbiol, 2013. 51(8): p. 2633-40.
- Starks, A.M., et al., Mutations at embB codon 306 are an important molecular indicator of ethambutol resistance in Mycobacterium tuberculosis. Antimicrob Agents Chemother, 2009. 53(3): p. 1061-6.
- Angra, P.K., et al., Performance of tuberculosis drug susceptibility testing in U.S. laboratories from 1994 to 2008. J Clin Microbiol, 2012. 50(4): p. 1233-9.
- APHL, Issues in Mycobacterium tuberculosis Complex Drug Susceptibility Testing: Ethambutol, in APHL Issues in Brief: Infectious Diseases. 2016, Association of Public Health Laboratories: Washington, D.C.
- Madison, B., et al., Multicenter evaluation of ethambutol susceptibility testing of mycobacterium tuberculosis by agar proportion and radiometric methods. J Clin Microbiol, 2002. 40(11): p. 3976-9.
- Zhang, Y. and W.W. Yew, Mechanisms of drug resistance in Mycobacterium tuberculosis: update 2015. Int J Tuberc Lung Dis, 2015. 19(11): p. 1276-89.
- Centers for Disease Control and Prevention, Treatment of Tuberculosis, American Thoracic Society, CDC, and Infectious Diseases Society of America. 2003, MMWR. p. 4,11,19-20.
- Whitfield, M.G., et al., The potential use of rifabutin for treatment of patients diagnosed with rifampicin-resistant tuberculosis. J Antimicrob Chemother, 2018. 73(10): p. 2667-2674.