Report of Expert Consultations on Rapid Molecular Testing to Detect Drug-Resistant Tuberculosis in the United States
Figure 1: Flow Chart of Steps in a molecular drug-resistance testing service
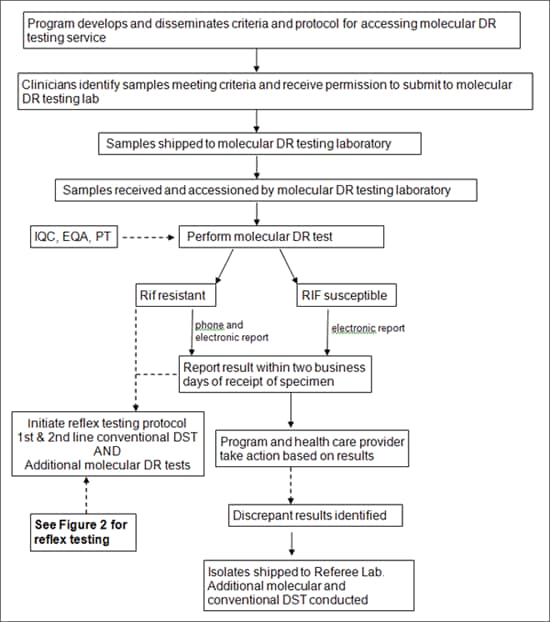
Figure 2: Reflex Testing
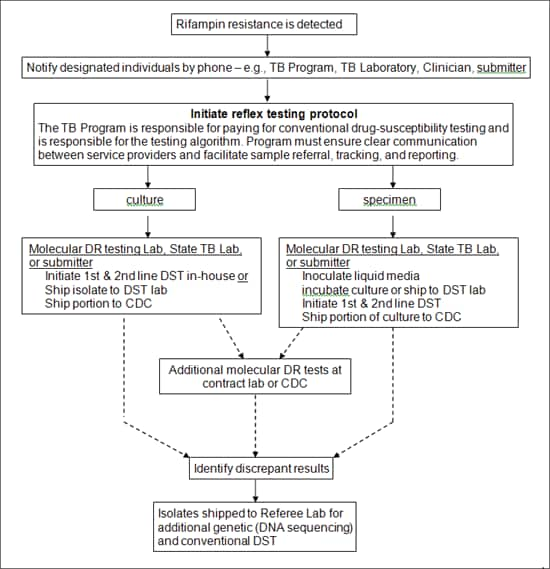
Figure1: Flow Chart of steps in a molecular drug-resistance testing service alternative text version
Program develops and disseminates criteria and protocol for accessing molecular DR testing service
<down arrow>
Clinicians identify samples meeting criteria and receive permission to submit to molecular DR testing lab
<down arrow>
Samples shipped to molecular DR testing laboratory
<down arrow>
Samples received and accessioned by molecular DR testing laboratory
<down arrow>
Perform molecular DR test <side input arrow>IQC, EQA, PT
<2 down arrows>
Rif resistant or RIF susceptible
Rif Resistant (phone and electronic report)
<2 down arrows>
Report result within two business days of receipt of specimen then/or Initiate reflex testing protocol 1st & 2nd line conventional DST AND Additional molecular DR tests
<up arrow into Initiate reflex testing protocol 1st & 2nd line conventional DST AND Additional molecular DR tests>
See Figure 2 for reflex testing
RIF susceptible (electronic report)
<down arrow>
Report result within two business days of receipt of specimen
<down arrow>
Program and health care provider take action based on results
<down arrow>
Discrepant results identified
<down arrow>
Isolates shipped to Referee Lab. Additional molecular and conventional DST conducted
Figure 2: Reflex Testing alternative text version
Rifampin resistance is detected
<down arrow>
Notify designated individuals by phone – e.g., TB Program, TB Laboratory, Clinician, submitter
<down arrow>
Initiate reflex testing protocol
The TB Program is responsible for paying for conventional drug-susceptibility testing and is responsible for the testing algorithm. Program must ensure clear communication between service providers and facilitate sample referral, tracking, and reporting.
<2 down arrows>
culture or specimen
Culture
<down arrow>
Molecular DR testing Lab, State TB Lab, or submitter
Initiate 1st & 2nd line DST in-house or
Ship isolate to DST lab
Ship portion to CDC
<2 down arrows>
Additional molecular DR tests at contract lab or CDC or Identify discrepant results
<down arrow>
Isolates shipped to Referee Lab for additional genetic (DNA sequencing) and conventional DST
Specimen
<down arrow>
Molecular DR testing Lab, State TB Lab, or submitter
Inoculate liquid media
incubate culture or ship to DST lab
Initiate 1st & 2nd line DST
Ship portion of culture to CDC
<2 down arrows>
Additional molecular DR tests at contract lab or CDC or Identify discrepant results
<down arrow>
Isolates shipped to Referee Lab for additional genetic (DNA sequencing) and conventional DST