Diagnosis & Detection
Clinicians: For 24/7 diagnostic assistance, specimen collection guidance, shipping instructions, and treatment recommendations, please contact the CDC Emergency Operations Center at 770-488-7100.
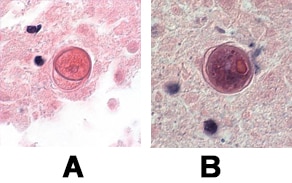
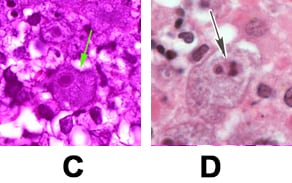
Initial testing: CSF typically demonstrates a predominantly lymphocytic pleocytosis with typically fewer than 500 cells/mm3. CSF glucose concentration may be normal or low and CSF protein concentration is elevated. Balamuthia organisms are rarely seen in the CSF. In the U.S. case series of 109 patients, only one patient was reported to have had amebas visualized on a wet mount of the CSF. This is in contrast to primary amebic meningoencephalitis caused by Naegleria fowleri where amebas are often visualized in the CSF of affected patients.
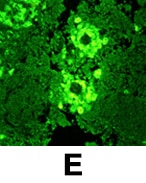
Balamuthia Case definition: In 2011, the Council of State and Territorial Epidemiologists (CSTE) established a standard case definition for Balamuthia infections. Laboratory-confirmed Balamuthia infection is defined as the detection of Balamuthia as:
- Organisms in CSF, biopsy, or tissue specimens, or
- Nucleic acid in CSF, biopsy, or tissue specimens, or
- Antigen in CSF, biopsy, or tissue specimens.
Tests available: Diagnostic testing is not widely available for Balamuthia infection. Clinicians who suspect Balamuthia infection should contact their state health department and/or CDC (24/7 Emergency Operations Center—770-488-7100). CDC can assist with diagnosis and provide treatment recommendations. Telediagnosis can be arranged at CDC by emailing photos through DPDx, CDC’s telediagnosis tool. Instructions for submitting photos through DPDx are available at the DPDx Contact Us page.
Diagnostic Tests
Diagnostic Tests Offered by CDC
Below are instructions for submitting specimens to CDC for free-living ameba testing. Please see the CDC Infectious Diseases Laboratory Test Directory for additional information.
Specimens can be sent to CDC for diagnostic assistance. If possible, please send the following specimens:
- Fresh, unfixed tissue or CSF
- Frozen samples, fresh unpreserved samples, samples reserved in ethanol or commercial fixatives designed to be compatible with molecular testing are suitable for PCR. If the specimen is preserved in formalin, CDC will still accept the specimen but note that formalin-fixed samples are not the preferred sample-type for molecular testing (e.g., PCR or real-time PCR).
Fresh, unfixed specimens (i.e., tissue and CSF) should be sent at ambient temperature by overnight priority mail. Fresh tissue sample should be sent in small volume (e.g., 0.5 mL) of equal volume mixture of sterile water and physiological saline to keep it moist. Please ship these specimens separately from other chilled or frozen samples being shipped.
Please send previously frozen tissue or CSF by overnight priority mail on ice packs (DO NOT ship on dry ice). Formalin-fixed (wet) tissues and formalin-fixed, paraffin-embedded (FFPE) tissue blocks should be shipped at ambient temperature.
Care should be taken to pack glass slides securely, as they can be damaged in shipment if not packed in a crush-proof container.
For fresh, unfixed specimens, frozen tissues, CSF, or H&E-stained or unstained slides only, please use the CDC specimen submission form pdf icon[PDF – 2 pages] and send to the address below. Be sure to add the pertinent travel history as well as relevant clinical history and any results from previous infectious disease testing. Please arrange Monday–Friday delivery only. Packages cannot be accepted on weekends or federal holidays.
CDC Shipping address:
CDC
SMB/STAT
Attn: Unit 53
1600 Clifton Road, NE
Atlanta, GA 30333
USA
See Specimen Submission Guidelines for Pathologic Evaluation of CNS Infections for more information.
- For submission of Formalin-fixed (wet) tissue or formalin-fixed paraffin-embedded (FFPE) tissue blocks
- Formalin-fixed (wet) tissues or formalin-fixed, paraffin-embedded (FFPE) tissue blocks
- Specimens should be submitted to CDC’s Infectious Diseases Pathology Branch (IDPB). Pre-approval is required. See this link for pre-approval and specimen submission procedures.
- Formalin-fixed (wet) tissues or formalin-fixed, paraffin-embedded (FFPE) tissue blocks
Telediagnosis can be arranged at CDC by emailing photos through DPDx, CDC’s telediagnosis tool. Instructions for submitting photos through DPDx are available at the DPDx Contact Us page.
Please contact the DPDx Contact Us page before collecting samples if further information or guidance is required.