NSSP Update – August 2019

- Community of Practice
- Workgroup and Committee Updates
- NSSP Community of Practice Call
- Implementation Guide for Syndromic Surveillance
- CDC Funding Recipients and Partnerships
- DoD–VDH Data-sharing Pilot
- Maricopa Co., Arizona, Uses SyS to Help Tribal Communities
- ESSENCE Queries
People
Community of Practice
Workgroup and Committee Updates
Technical Committee (TC): The TC assembled a small group of volunteers to review and comment on proposed feature enhancements in ESSENCE. This group reviewed some new filters in the ESSENCE Query Portal that would allow users to limit their query results based on Chief Complaint or Discharge Diagnosis availability. The next TC meeting is slated for October 3 at 2:00 PM ET. The leadership group is reviewing and prioritizing enhancement requests to determine the agenda for the next meeting. They will also review responses from the ESSENCE Chief Complaint Processing, Binning, and Business Logic Changes survey to determine recommendations for ESSENCE changes and next steps. Additionally, Caleb Wiedeman has agreed to serve with Natasha Close as co-chair of this committee.
Monthly NSSP CoP Call
Please join your colleagues for the monthly NSSP Community of Practice (NSSP CoP) call. The next call will be held August 27, 2019, 3:00–4:30 PM ET. This month’s topic is PEP this…Syndromic Surveillance and Rabies PEP Administration.
New member? Syndrome? Success? Email NSSP!
Be part of the NSSP Community of Practice (CoP). To participate, please email nssp@cdc.gov and give your name, contact information, and request to be part of the NSSP CoP. If you want to join a committee or workgroup, we will forward your email to that group’s chair and co-chair.
If you have a syndrome to share, success story to showcase, or resources to post on the Knowledge Repository, email nssp@cdc.gov. We will forward your request to the appropriate NSSP CoP committee or workgroup to review before posting.
Implementation Guide for Syndromic Surveillance
Final edits of the Implementation Guide for Syndromic Surveillance Release 1.0 were completed in June 2019 ahead of schedule, thanks to participation of Community of Practice members. Last month, the guide was posted on the HL7 website, and the National Institute of Standards and Technology Implementation Authoring Tool (NIST IGAMT) released the message validation tool for testing. NIST IGAMT is open access, and anyone can enter the site as a guest user and validate messages for this specification.
The ISDS and CDC Workgroup completed its revision of the Implementation Guide. The HL7 Public Health Workgroup approved the guide, launching a 90-day period for review so that all HL7 members have sufficient time to comment. The Implementation Guide will be posted as a WIKI page for collaborative editing. If you aren’t an HL7 member, you can make suggestions by submitting a ticket to the NSSP Service Desk (an account is required). NSSP has set up an Implementation Guide queue to triage comments. The WIKI is located here and will be available to the general public for download on October 26, 2019. The 90-day comment period closes November 1, 2019.
| HL7 2.5.1 Implementation Guide Milestones* | |
|---|---|
| Time Frame | Activity |
| 2015 | Completed Version 2.0 Final RELEASE** |
| 2016 | Released Erratum and Clarification Documents for Version 2.0 |
| 2017 Summer | Released Version 2.2 Working Draft for Community Comment and Consensus |
| 2017 Winter | Released Version 2.3 for Review and Community Comment |
| 2018 March | Released Version .09 |
| 2018 Spring | Submitted DRAFT HL7 Guide for Balloting: Implementation Guide for Syndromic Surveillance Release 1.0 Standard for Trial Use (STU) HL7 Version 2.5.1 |
| 2018 Fall (October–December) |
|
| 2018 November– 2019 March |
|
| 2019 January | Provided Final Resolution for 33 Comments (Pending) |
| 2019 February |
|
| 2019 March–May |
|
| 2019 June 20–27 | Hand-off to HL7 Public Health Workgroup for 2-week Review |
| 2019 July |
|
| 2019 Summer (June–August) |
|
| October 26, 2019 | Release to Public: HL7 2.5.1 Implementation Guide for Syndromic Surveillance for Trial Use Version 1 as a Standard for Trial Use for the General Public |
| 2019 September–November |
|
| 2019 September–2022 September | Publish Standard for Trial Use (STU) for Trial Period of 1 to 3 years (during which time additional comments may be submitted and dispositioned and additional releases of the guide published) |
* Milestones revised July 31, 2019.
** Version 2.0 is currently being used; subsequent versions are working drafts only.
Shaded activities have been completed.
Fall 2019 Pilot Testing of Implementation Guide

Let’s kick the tires and take the Implementation Guide for a spin. In the next few months, NSSP will lead pilot tests for EHR vendors, hospitals, and health departments. About 30 variables were revised or created, and the pilot will assess whether business processes across these organizations can support the new guidance with reasonable effort.
This document will guide the community’s efforts for years to come. If you would like to volunteer, we’re beginning the planning process and scoping out the evaluative measures for the pilots. We anticipate the pilots to begin in the fall. We could use your help making sure messages are complete, processed seamlessly, and received intact. More details will be shared soon. If interested, please email nssp@cdc.gov.
CDC Funding Recipients and Partnership Updates
DoD–VDH Data-sharing Pilot

The Department of Defense (DoD) and the Virginia Department of Health (VDH) maintain local ESSENCE systems to monitor the health status of their military and civilian populations, respectively. In June 2018, DoD and VDH collaborated on a pilot project to develop processes for data sharing, data access, and communication. Their goal was to develop best practices that states could use to share syndromic surveillance data with DoD using ESSENCE through CDC’s National Syndromic Surveillance Program (NSSP).
For the first time, DoD and civilian syndromic surveillance data were visible within the same system, at the same time. Epidemiologists at VDH observed DoD operations and could see potential reportable-condition cases that might not be reported to local public health authorities. DoD participants observed disease trends outside an installation, gaining information that could better prepare military health personnel to respond to outbreaks and emerging infectious disease.
Overall, the pilot strengthened their working relationship and provided the opportunity for collaborative public health surveillance and response. In one instance, for example, a DoD public health practitioner alerted colleagues at the local health department of an outbreak of influenza-like illness at an elementary school on the military installation. Through data sharing, DoD and VDH visualized the event and monitored it in the context of other illnesses affecting the local community. VDH and DoD supported the school by monitoring influenza-like illness in the surrounding county and by recommending control measures.
DoD and VDH continue to share post-pilot data and have regular discussions about NSSP data. Once DoD transitions to the next version of ESSENCE, DoD health practitioners plan to expand data sharing to other states. The ability to view DoD and civilian health encounter data in the same system is a monumental step in responding promptly to outbreaks and emerging infectious disease that affect civilian and military populations.
Maricopa County, Arizona, Uses SyS to Help Tribal Communities
Last month we shared Maricopa County Department of Public Health’s (MCDPH) success with using syndromic surveillance to monitor the transfer of patients with suspected Rocky Mountain Spotted Fever (RMSF). Since then, we’ve added MCDPH’s story to the NSSP website. If you aren’t familiar with the story, take a moment to read about how analysts in Arizona use syndromic surveillance to complement public health protocols that rely on passive surveillance. They’ve successfully used syndromic data to improve health care for tribal communities, a population historically underserved with poorer health outcomes compared with non-tribal communities in the United States.
ESSENCE QUERIES
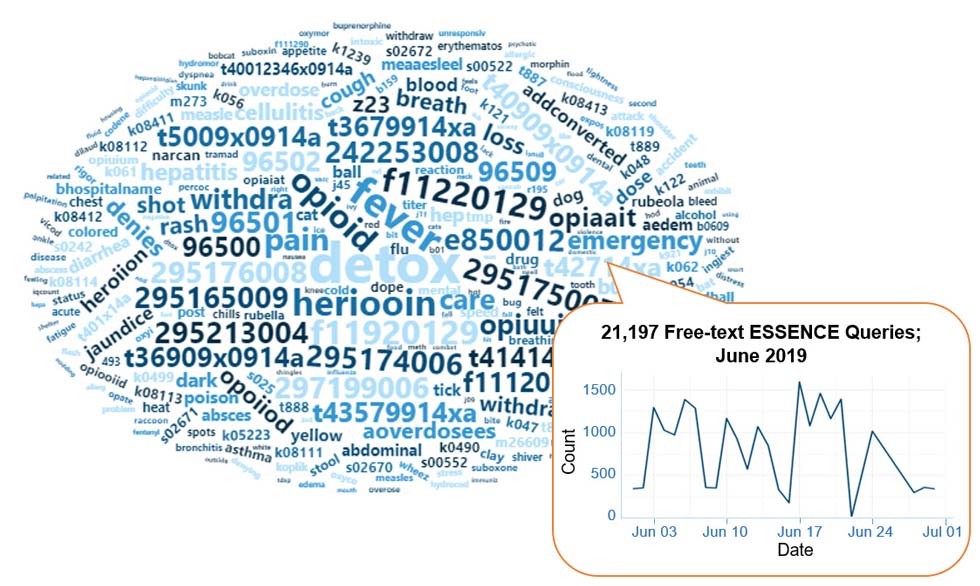
Query Terms from June 2019
A key feature of NSSP–ESSENCE is its ability to use free-text definitions across different fields to generate custom queries. The SyS community can use this feature to develop and refine category definitions and to identify and respond to public health events that may not be apparent in established categorical definitions (i.e., syndromes, subsyndromes, and Chief Complaint and Discharge Diagnosis [CCDD] categories).
This word cloud summarizes free-text and coded values that epidemiologists and data analysts queried in ESSENCE during June 2019. This word cloud was generated using the Wordcloud2 package in RStudio. The relative size of the terms suggest the frequency of use in a query. NSSP super administrators can query ESSENCE usage logs to learn what queries are run nationwide and how long they take to complete.
Practice
Collaborations Accelerate SyS Practice

In 2019, something at CDC and across health departments clicked. Analysts, health scientists, and epidemiologists saw opportunities to join forces in unprecedented numbers. We saw an acceleration in collaborative projects across areas in which syndromic surveillance had not been used before. If you follow NSSP CoP workgroup and committee activities and read the newsletter, you see many awe-inspiring ways in which collaborations are advancing science. We want to highlight some of these collaborations that are rapidly moving us into 2020:
Three-month Outlook:
- Implement syndrome definition for medication refill for during and after a public health disaster.
- Develop syndrome definition for pregnancy, delivery, and miscarriage.
- Collaborator: CDC’s Maternal and Infant Health/Maternal and Infant Health Branch (MIHB)
- Scheduled for early fall 2019
- Develop syndrome definition for alcohol-involved emergency department visits.
- Collaborator: Opioid and Overdose Team
- Scheduled for early fall 2019
- Develop syndrome definition for injecting drug users (IDUs) and endocarditis (infection of inner lining of heart chamber and valves).
- Collaborator: CDC’s National Center for Emerging and Zoonotic Infectious Diseases, Epidemiology Research and Innovations Branch
- Scheduled for fall 2019
- Develop syndrome definition for motor vehicle collision (e-scooters). NSSP is part of the Transportation Safety team, e-scooter working group.
2019–2020:
- NSSP is participating in a functional exercise to gauge how well the community shares information and data during a public health event. This exercise will evaluate response speed; technical needs and delivery options; potential local, state, and federal collaborations; and integration of emergency preparedness and management personnel.
- Collaborator: NSSP CoP SyS Public Health Emergency Preparedness Response and Recovery (SPHERR)
- The NSSP team is working with the community and CDC’s National Center for Injury Prevention and Control (the Injury Center) to re-categorize sexual violence syndromes into broad and narrow concepts that better use syndromic data.
- NSSP is working with CDC’s National Center for Chronic Disease Prevention and Health Promotion, Division of Cancer Prevention and Control, on a special-interest project though the Prevention Research Centers Program: “Improving Cancer Survivor Treatment and Outcomes by Ensuring Appropriate Emergency/Acute Care Treatment.” Work will begin fall 2019 and extend through 2020. (Note: NSSP also collaborated on “Symptoms and Characteristics of Cancer Patient Visits to an Emergency Room,” which was published online in the Journal of Clinical Oncology on May 26, 2019. DOI: 10.1200/JCO.2019.37.15_suppl.e18306.)
- The NSSP team is developing a syndrome definition for ketoacidiosis (a life-threatening but avoidable complication of diabetes when high levels of blood acids, ketones, are produced), specifically searching the discharge diagnosis field for ICD-9/10 codes.
- Collaborator: Division of Diabetes Translation with their Surveillance Team
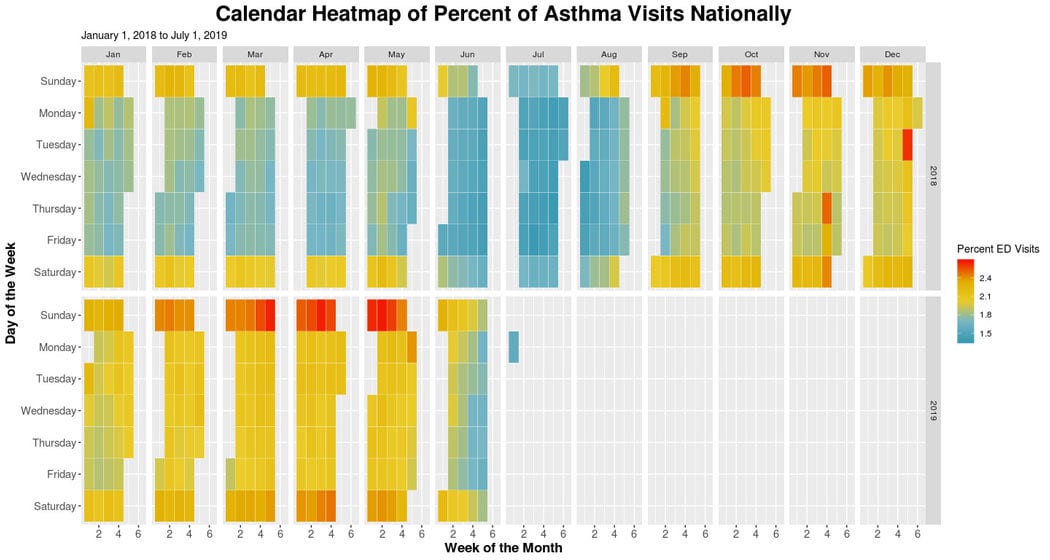
Calendar Heatmaps for Visualizing Time-series Data
A calendar heatmap can be used to visualize time-series data and provide more information than a conventional line chart time series. It can help detect patterns, anomalies, or trends by day of week, week of the month, months, and years. By design, heatmaps incorporate vast amounts of data that could be challenging for an audience to analyze and interpret. Yet it takes but a moment to fully grasp the information. For this reason, heatmaps have been useful in analyzing emergency department data to identify a syndrome’s seasonal trends, patterns, and anomalies. The NSSP team is in the early stages of using heatmaps to analyze syndome trends.
Take a look, for example, at the calendar heatmap below that we created using R (SAS and other programs may be used, too). This heatmap summarizes asthma visits across the nation, allowing us to observe a few different trends. We notice a monthly seasonality of asthma-related visits. During the summer months, a lower percentage of visits related to asthma occur. Additionally, during the first five months of 2019, we observe a higher percentage of emergency department visits than in 2018. Perhaps this is due to the discharge diagnosis field becoming more complete over time or to new facilities being onboard. Either factor could influence the high percentage of asthma-related visits in 2019 compared with 2018. We can also determine a day-of-week trend, with the highest percentage of visits occurring on Saturdays and Sundays regardless of the month.
Free-text Coding in NSSP–ESSENCE
This is the fourth in a series of articles about how to write ESSENCE free-text queries.
“Part 1. Wildcards” can be found here.
“Part 2. Underscores_and Brackets [ ]” can be found here.
“Part 3. Inclusionary Terms” can be found here.
Introduction

The search criteria for ESSENCE free-text queries are built around Boolean logical operators and regular expressions. Free-text queries are not case-sensitive and may contain “^” for wildcards; “,” for multiple entries; “ISBLANK” to look for blanks; “ISNULL” to look for nulls; [COMMA] to look for commas; and operators “and,” “or,”, “andnot,” and parentheses “()” to define order and grouping. This series will cover all these topics in-depth.
Free-text queries are what makes syndromic surveillance practice, particularly practice using NSSP–ESSENCE, adaptable to different data sources and types. By using free-text queries, analysts and epidemiologists can exercise a high level of customization. They can quickly code free-text queries and rapidly respond to outbreaks, disasters, and events that unfold. Such capabilities empower users to customize queries to fit their level of data, ensuring accurate results.
Free-text coding in ESSENCE, which is accessible to all users, follows distinct patterns. Learning to read these patterns allows users to take queries from many places and repurpose them to suit their unique needs. Syndromic surveillance depends heavily on sharing methods, and practitioners must understand the language.
Part 4. Exclusion Terms and Parentheses
So far in this series, we have covered basic query notation, use of Carets, Underscores, and Brackets, and “OR” and “AND” statements. The final two parts of a query’s structure can involve “ANDNOT” statements and parentheses. We have demonstrated the power of inclusion terms, and the “ANDNOT” statement is the next step to leveraging the system’s query resources. We will also show how to add parentheses to specify how these “OR”, “AND”, and “ANDNOT” statements are applied and interact with each other.
“ANDNOT” statements
“ANDNOT” statements, also referred to as negations, exclusions, or exclusionary statements, are a common way to specify what you do not want your query to return. These statements are often leveraged in a syndrome definition to exclude common false positives. An “ANDNOT” statement without parentheses connects the word before it and the word after and will remove any returns where both criteria are met. To demonstrate this, we can work through the following very simple query.
THIS,OR,THAT,ANDNOT,THERE
In this query, ESSENCE will return fields containing the term “THIS” or the term “THAT” and then look through the “THAT” returns for the presence of the term “THERE” and remove these records where both criteria are met. “ANDNOT” statements do not specify the order in which the terms appear. Also note that the example’s “THERE” exclusion is only applied to “THAT” and does not apply to “THIS”. “THIS” is unaffected by the exclusion statement. Similar to “AND” and “OR” statements, ESSENCE queries are not case sensitive, neither are the operators, and all operators should be surrounded by commas.
Let’s assume the following Chief Complaints (used in Parts 1–3) and a desire to create a query for fall-related injuries.
- Fall
- Fell getting out of car
- Left arm injury; Fall
- Falling out with friends; Suicidal
- Feels crestfallen
- Patient brought in after falling on face
- Fall; Left wrist injury
- Feels congested; Allergies
You may reasonably assume the boldfaced CCs 1, 2, 3, 6, and 7 are the intended cases and 4, 5, and 8 are false positives.
Here’s a table that shows examples of the “ANDNOT” query:
| “ANDNOT” Query Examples | |
|---|---|
| Code | Description |
^Fall^ |
This is a simple query of a term surrounded by carets taken from Part 1. It returns CCs 1, 3, 4, 5, 6, and 7. |
^Fall^,ANDNOT,^Crestfallen^ |
This query negates the false positive in CC 5. Returns CCs 1, 3, 4, 6, and 7. |
^Fall^,OR,^Fell^,ANDNOT,^Crestfallen^ |
This covers both forms of “Fall” in our CCs, but the negation of “Crestfallen” follows the term ^Fell^. Since “Fell” and “Crestfallen” don’t appear in the same CC, no CCs are negated. Returns CCs 1, 2, 3, 4, 5, 6, and 7. |
^Fall^,ANDNOT,^Falling out^,ANDNOT,^Crestfallen^ |
“ANDNOT” statements can be written in series. Multiple exclusionary statements in a row apply to the inclusionary term they immediately follow. Both negation terms apply to the ^Fall^ term. Returns CCs 1, 2, 3, 6, and 7. |
Parentheses
Complex syndrome definitions often use parentheses to group terms and apply statements in different ways. “ANDNOT” statements are frequently used alongside parenthetical groupings. Regarding notation in ESSENCE, all parentheses should be surrounded by commas, except when the parenthesis starts or ends the statement. To demonstrate this notation and how parentheses can redistribute statements, we can work through the following simple query.
(,THIS,OR,THAT,),ANDNOT,(,THERE,OR,THEN,)
In this query, since both sections are surrounded in parentheses, ESSENCE will apply both the “ANDNOT” negations “THERE” and “THEN” to both the initial inclusion terms “THIS” and “THAT”. That means it will return fields containing either term “THIS” or the term “THAT” so long as there is not also the presence of either term “THERE” or “THEN”. Notice the second “(“ is surrounded by commas while the first one isn’t since it is the first character in the query. Likewise, notice the first “)” is surrounded by commas while the last one isn’t since it’s the last character in the query. Misplaced commas are one of the most frequent reasons a query doesn’t work as intended.
Let’s assume the following Chief Complaints (used in Parts 1–3) and a desire to create a query for fall-related injuries.
- Fall
- Fell getting out of car
- Left arm injury; Fall
- Falling out with friends; Suicidal
- Feels crestfallen
- Patient brought in after falling on face
- Fall; Left wrist injury
- Feels congested; Allergies
You may reasonably assume the boldfaced CCs 1, 2, 3, 6, and 7 are the intended cases and 4, 5, and 8 are false positives.
Here’s a table that shows examples of queries with parentheses:
| Query Examples with Parentheses | |
|---|---|
| Code | Description |
^Fall^ |
This is a simple query of a term surrounded by carets taken from Part 1. It returns CCs 1, 3, 4, 5, 6, and 7. |
(,^Fall^,) |
Sometimes parentheses won’t change anything. This query functions exactly the same as the query above it. Returns CCs 1, 3, 4, 5, 6, and 7. |
(,^Fall^,OR,^Fell^,),ANDNOT,^Crestfallen^ |
This covers both forms of “Fall” in our CCs, and the parentheses ensure the negation applies across both initial terms. Returns CCs 1, 2, 3, 4, 6, and 7. |
^Fall^,ANDNOT,(,^Falling out^,OR,^Crest fallen^,) |
“ANDNOT” statements can be written and applied to a series of “OR” statements, excluding the whole statement in the parentheses. Returns CCs 1, 2, 3, 6, and 7. |
(,^Fall^,OR,^Fell^,),ANDNOT,(,^Cre stfallen^,or,^Falling Out^,ANDNOT,(,^Out a window^,),) |
Syndromes can get complicated with multiple-nested parentheses. Notice this query attempts to negate the “Falling out with friends” CC while also trying to include visits related to “Falling out a window.” In this instance, the “ANDNOT” term is double negated by a second “ANDNOT” term. Returns CCs 1, 2, 3, 6, and 7. |
Putting two sets of parentheses with different criteria linked by an “AND” statement means at least one item from each set of criteria must be met. In the example below, both coded statements will return the same visits, but the second statement is far more concise and easier to read.
^Fall^,AND,^Injur^,OR,^Fall^,AND,^Face^,OR,^Fell^,AND,^Injur^,OR,^Fell^,AND,^Face^
(,^Fall^,or,^Fell^,),AND,(,^Injur^,or,^Face^,)
Here are a couple examples to show how “ANDNOT” and parentheses can be used in practice:
Example 1:
^measl^,ANDNOT,^titer^,ANDNOT,^measles mumps rubella^,ANDNOT,^mmr^,ANDNOT,^vacc^,ANDNOT,^shot^,ANDNOT,^immun^,ANDNOT,^rubel^,ANDNOT,^proph^,ANDNOT,^room b05^,ANDNOT,^german^,ANDNOT,^homesless^,ANDNOT,^rule out measles^,OR,^meez^,ANDNOT,^titer^,ANDNOT,^measles mumps rubella^,ANDNOT,^mmr^,ANDNOT,^vacc^,ANDNOT,^shot^,ANDNOT,^immun^,ANDNOT,^rubel^,ANDNOT,^proph^,ANDNOT,^room b05^,ANDNOT,^german^,ANDNOT,^homesless^,ANDNOT,^rule out measles^,
Example 2:
(,^[;/ ]T40.1X1A^,OR,^[;/ ]T401X1A^,OR,^[;/ ]T40.1X4A^,OR,^[;/ ]T401X4A^,OR,^[;/ ]965.01[;/]^,OR,^[;/ ]96501[;/]^,OR,^[;/ ]E850.0^,OR,^[;/ ]E8500^,or,^295174006^,or,^295175007^,or,^295176008^,),or,(,(,(,^narcan^,or,^naloxo^,or,^poison^,or,^verdo[se][se]^,or,^over dose^,or,^overose^,or,^nodding^,or,^ nod ^,or,^snort^,or,^in[gj]est^,or,^intoxic^,or,^unresponsiv^,or,^loss of consciousness^,or,^syncop^,or,^shortness of breath^,or,^short of breath^,or,^altered mental status^,),and,(,^her[io][oi]n^,or,^ hod ^,or,^speedball^,or,^speed ball^,or,^dope^,),),andnot,(,^no loss of consciousness^,or,^denie[sd] loss of consciousness^,or,^negative loss of consciousness^,or,^denies any loss of consciousness^,or,^denies her[io][oi]n^,or,^deny her[io][oi]n^,or,^denied her[io][oi]n^,or,^denying her[io][oi]n^,or,^denies drug^,or,^deny drug^,or,^denied drug^,or,^denying drug^,or,^denies any drug^,or,^with dra^,or,^withdra^,or,^detoxification^,or,^detos^,or,^detoz^,or,^dtox^,),)
This example is the entire CDC Heroin Overdose v4 CCDD Category. Notice how the green statement is linked to the yellow by an “AND” statement. Then, both statements are contained within parentheses and negated by the red statement with an “ANDNOT” operator.
Try it out!
Compare the results of the following queries in the CCDD field of ESSENCE (results should be exactly the same):
^Fall^,AND,^W19.XXXA^,OR,^Fall^,AND,^W19XXXA^
^Fall^,AND,(,^W19.XXXA^,OR,^W19XXXA^,)
Try writing a set of negation terms (expected false positives) for the following query. Check your answer with the CDC Pertussis v1 CCDD Category in NSSP ESSENCE.
(,Whooping^,OR,^Pertuss^,),ANDNOT,(_______________)
We thank Senior Data Analyst Zachary Stein for volunteering to write a series of articles about free-text coding. Stein, formerly with the Kansas Department of Environment and Health, does epidemiologic work to support NSSP efforts. Stein is an active participant in the NSSP CoP. He initially wrote about free-text coding as an entry on the NSSP CoP Syndrome Definition Committee forum. The forum generated considerable interest, inspiring this series. Stein acknowledges input provided by others who contributed to the forum post.
Data Quality Corner
Updated NSSP Data Dictionary Now Available: ESSENCE APIs, ESSENCE Daily Availability Indicator, and More!
You asked for it, and we delivered! Check out the updated NSSP Data Dictionary. It offers several enhancements to help you make the most of ESSENCE. Here’s what’s new:
ESSENCE API and Data Details Worksheet
This worksheet includes information on application programming interfaces (APIs) available through ESSENCE and gives details about data served through the APIs.
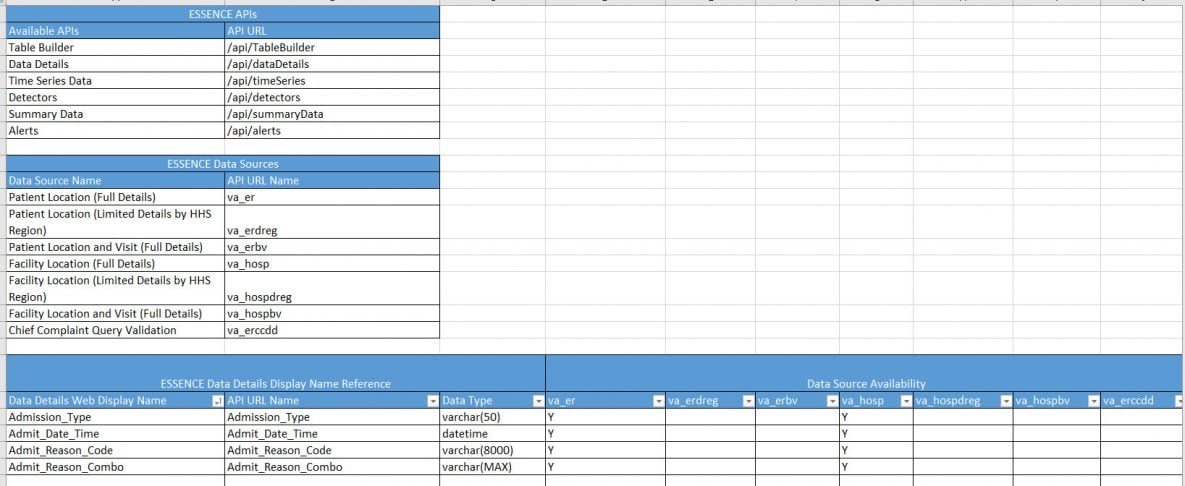
What’s included?
- Table of available ESSENCE APIs
- Table of commonly used ESSENCE data sources and API data source names:
- Patient Location (Full Details)
- Patient Location (Limited Details by HHS Region)
- Patient Location and Visit (Full Details)
- Facility Location (Full Details)
- Facility Location (Limited Details by HHS Region)
- Facility Location and Visit (Full Details)
- Chief Complaint Query Validation
- Table of ESSENCE Data Details (API name references, data source availability)
ESSENCE API Query Parameters Worksheet
This worksheet lists query parameter naming conventions and data source availability.
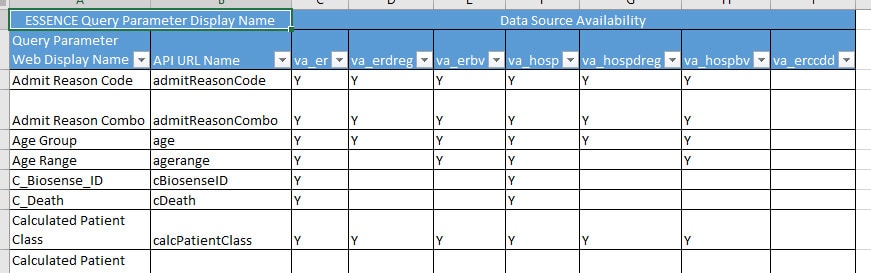
What’s included? The Table of ESSENCE Parameters includes ESSENCE query parameter display name, API URL, data type, and data source availability for query parameters.
ESSENCE Worksheet
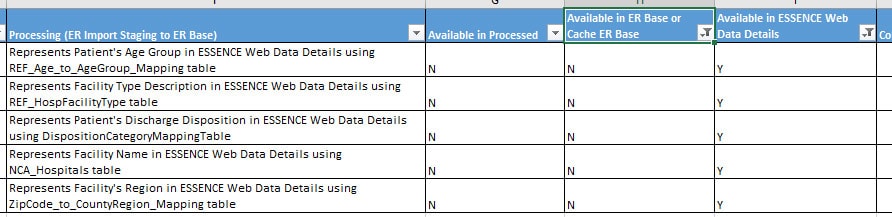
The original design of the ESSENCE worksheet described data ingestion from the core NSSP Processed data to a collapsed version of the visit record into ESSENCE’s Cache ER Base table. Over time, we added information about calculated fields and inferred data elements such as Disposition_Category.
Some data elements are available in NSSP Processed data but are not ingested into ESSENCE. Others are available in the “Data Details” display but are not stored in Cache ER Base. Still others are stored in Cache ER Base but are not included in “Data Details.” And yes, knowing what is available and where can be confusing.
That’s why we added indicators in the ESSENCE worksheet to note availability of data in Processed, Cache ER Base, and Data Details. Check out the new columns labeled “Available in Processed,” “Available in ER Base or Cache ER Base,” and “Available in ESSENCE Web Data Details:
NICC Terms Worksheet
A list of Non-Informative Chief Complaint (NICC) terms has been added to the Dictionary. If you need a refresher on NICC terms and use, see last month’s newsletter. The same NICC terms that designate informative versus not informative information for chief complaint now apply to discharge diagnosis, too.
More Dictionary Worksheet Updates
- ArchiveProcessed
- Updated MessageDate processing to include description of proposed processing change when MessageDate occurs before C_Visit_Date (ID #60)
- Deleted “Concatenate repeating segments” and clarified processing of Initial_Temp to indicate that data element will be NULL if Str_Initial_Temp has concatenated values (ID #102)
- Exceptions_Reason:
- Updated the description of Exceptions_Reason_Code 11 to clarify Arrived_Date_Time processing and include information about Message_Date processing
- ESSENCE:
- Updated Initial_Temp processing description to include how data element can take on concatenated values; deleted “concatenate the first complete pair” to clarify that no “take first value” processing logic is applied (ID #102: Column E)
- Updated Initial_Temp_Calc processing description to reflect new ESSENCE temperature value range mapping logic (ID #102: Column F)
- Updated DeathIndicator so that it is populated by using the value from Patient_Death_Indicator (ArchiveProcessed) (ID #60)
- Added new ESSENCE data element MessageDate processing to describe pre-existing error when MessageDate occurs before C_Visit_Date (ID #272)
- Added new ESSENCE Data Elements: DDInformative (ID #266), CCInformative (ID #32), and CCAvailable (ID #95)
Data Quality on Demand Now Available through SAS Studio

Interested in making your own detailed NSSP production data quality reports for Completeness, Timeliness, and Validity? Want to control the visit date range in reports? You can do both using the DataQuality_OnDemand.sas (DQOD) SAS program.
DQOD is part of a suite of programs available in NSSP SAS Studio. DQOD puts more control in your hands. Launch DataQuality_OnDemand.sas to run one or more reports for any date range and produce Excel files with the same content as the detailed DQ reports NSSP releases each month. Simply copy the main program, DataQuality_OnDemand.sas, update a few settings, and go!
A Quick Start Guide has been developed and will be posted in the NSSP Resource Center soon. Meanwhile, please contact the service desk for help.
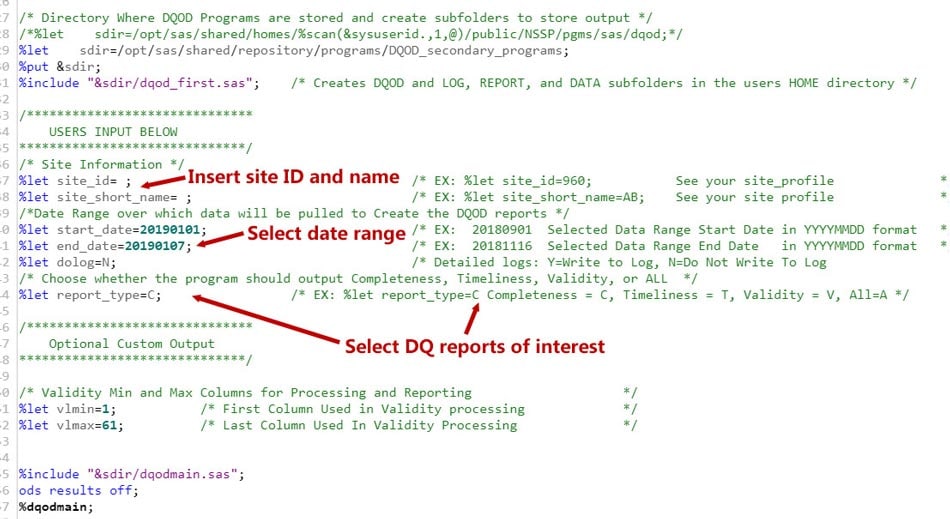
How to Use DQOD
First, locate the SAS Studio tool. Under Programs, scroll to the code for DataQuality_OnDemand.sas. Once there, select the options you want (shown below), and then run the program:
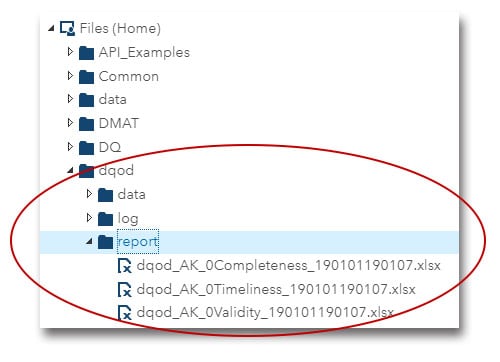
The reports will be delivered to your “dqodreport” directory:
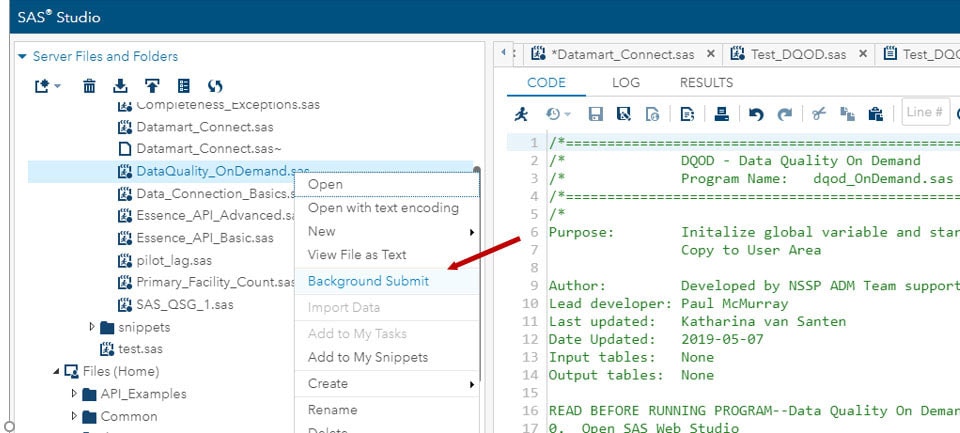
As a reminder, you can “Background Submit” your SAS jobs by right-clicking the program and making this selection from the pop-up window:
Click the menu button on the top navigation bar to see the status of your job:
Check Your Daily Site Processing Summary!

The “Daily Summary” is an automated email sent to site administrators and their designees each morning. It provides a snapshot of processing metrics and issues and is designed to help site administrators identify potential data processing issues quickly. These emails contain collated details on the data’s journey through various processing steps.
The Daily Summary delves into data processing (e.g., filtered records, exceptions, production backlogs) and summarizes facility activity, including alerts and volume anomalies. In addition, the Monday email displays anomalies associated with visit and message volume.
A quick scan of the email will show the status of your site’s data. For in-depth guidance, NSSP is developing a Quick Start Guide that will be posted in the Resource Center. The Guide will describe each section and how data are calculated. If you have questions, suggestions for improving the Daily Summary, or concerns about data processing, please submit a Service Desk ticket to support.syndromicsurveillance.org.
AMC Update Scheduled for Fall 2019
The NSSP team is improving the functionality of Data Access Rules, accessible via the Access & Management Center (AMC). The Data Access Rule tab will be redesigned to streamline the way in which rules are created and edited. The fall 2019 update will also include bug fixes for data sharing.
One-stop Shop for SAS Code that Supports ESSENCE APIs
NSSP has improved the SAS program for all ESSENCE application programming interface (API) options, including JSON, CSV, and PNG. The program is named ESSENCE_API_Basic.sas and can be found in the SASprograms directory.
The program accepts all ESSENCE API output types to produce a dataset that you can use in SAS Studio. Simply paste the ESSENCE API URL into the program, provide the name of the SAS dataset to create, and provide the ESSENCE API option being called (JSON, CSV, or PNG).
Current Month and Upcoming Events
| August 7 | Data Validation Conference Call, 3:00–4:00 PM ET |
| August 20 | Scheduled vendor patches in staging environment: 6:00–10:00 AM ET |
| August 22 | Scheduled vendor patches in production environment: 6:00–10:00 AM ET |
| August 27 | NSSP CoP Call; 3:00–4:30 PM ET; topic: PEP this…Syndromic Surveillance and Rabies PEP Administration |
Last Month’s Technical Assistance
| July 3 | Held Data Validation Conference Call |
| July 16 | Applied vendor patches in staging environment |
| July 18 | Applied vendor patches in production environment |
| June 27 | Updated the Access & Management Center |
NSSP Participation
A total of 58 sites from 46 states and the District of Columbia participate in NSSP. Currently, there are 4,478 facilities, including 3,021 emergency departments (EDs), actively contributing data to the NSSP BioSense Platform. Data from these EDs cover about 68% of all ED visits in the country.
NSSP publishes data for ED visit coverage each quarter. These data and the coverage map shown below were updated July 2019. The calculation method is described in the December 2018 issue of NSSP Update.
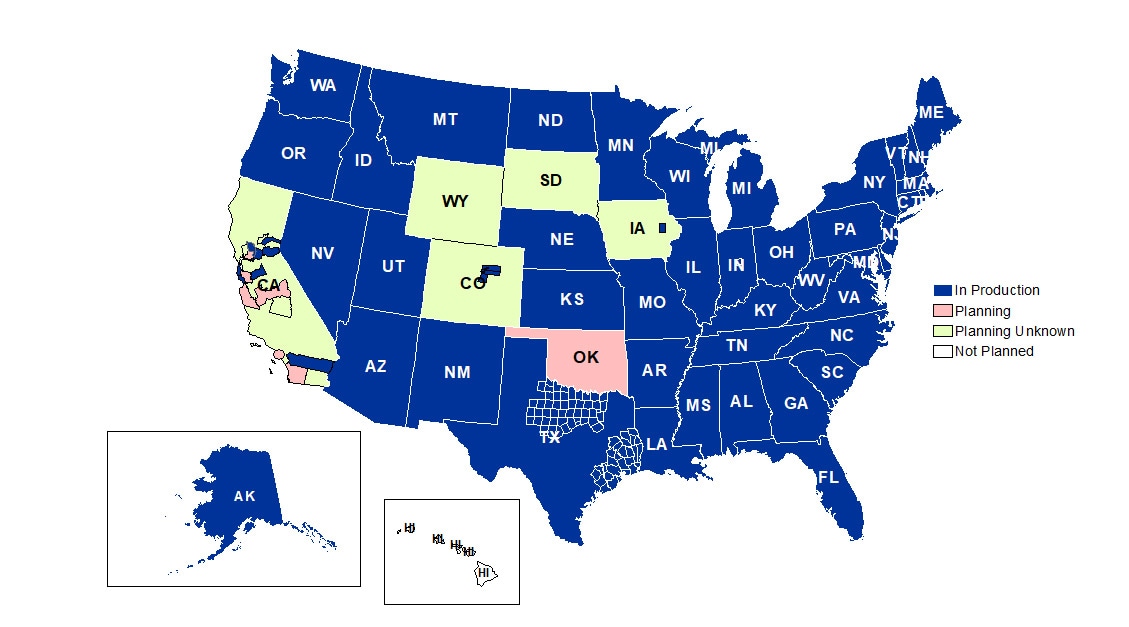
Definitions: NSSP consolidates facilities that provide data under a single data administrative authority called a site administrator. These facilities and single-site administrator constitute a site.


