February 2023
February 21, 2023, 3:00–4:00 PM ET: February eSHARE Webinar: “Case Surveillance Data Standardization—Paving the Way for Increased Interoperability.”
June 25–29, 2023: 2023 Council of State and Territorial Epidemiologists (CSTE) Annual Conference in Salt Lake City, UT. Register for the conference.
CDC and CSTE collaborating to standardize case surveillance data elements
Join CDC and the CSTE Data Standardization Work Group at the next eSHARE training webinar on February 21, 2023, 3:00 PM—4:00 PM ET. The webinar, titled “Case Surveillance Data Standardization—Paving the Way for Increased Interoperability,” will provide information about CDC’s and CSTE’s collaboration to:
- identify and define a standardized set of core data elements to be used across the case surveillance ecosystem for both infectious and non-infectious conditions and
- use these data elements to build standardized data dictionaries for case surveillance interoperability.
During the webinar, attendees will have the opportunity to ask questions and provide feedback to inform the proposed approach.
As part of larger efforts to modernize case surveillance, CDC and the CSTE Data Standardization Work Group are partnering to bring together jurisdictions, CDC programs, and other case surveillance experts. These discussions will inform recommendations for standardized case surveillance data elements aligned with existing data standards such as United States Core Data for Interoperability (USCDI).
This effort aims to:
- create standards for data across the case surveillance ecosystem for both infectious and non-infectious conditions;
- benefit jurisdiction surveillance systems and processes, and promote sharing of data among state, tribal, local, and territorial public health jurisdictions;
- coordinate with the CDC emergency response case data element project; and
- inform the new data dictionary for case notification to CDC.
This collaboration has already led to progress on standardizing case surveillance data elements. For instance, the CSTE Data Standardization Work Group recently developed a standardized approach that jurisdictions and CDC programs can use to assign dates for counting cases. Read the issue brief.
Contact Becky Lampkins at blampkins@cste.org to join the CSTE Data Standardization Work Group or if you have questions or feedback for CSTE.
Contact Danielle Tack at dot7@cdc.gov if you have questions or feedback for CDC.
CDC provides virtual training and consultation on NBS
CDC recently hosted two virtual events on the National Electronic Disease Surveillance System Base System (NBS) for programmatic and technical staff working at the state, tribal, local, and territorial levels. The events provided public health jurisdictions with a closer look at how NBS can help them manage their reportable disease data, and CDC staff were able to gain a deeper understanding of jurisdictions’ data systems, surveillance workflows, and modernization efforts.
Virtual training
The first event, a virtual training held January 17–20, 2023, provided comprehensive guidance on how to use NBS for public health surveillance. Topics included:
- surveillance workflows,
- data collection forms,
- interoperability with electronic laboratory reporting (ELR) and case reporting (eCR),
- reporting and analysis functionality,
- system monitoring and best practices, and
- case notification and data reconciliation processes.
An average of 150 people participated in each session, with some sessions hosting close to 200 participants. Attendees represented diverse organizations, including public health departments, NBS support vendors, and CDC programs.

The NBS team received positive feedback from attendees. As one participant noted, “You all were very organized and put together thorough, informative presentations covering many applicable topics. It is difficult to try to reach participants with a wide range of knowledge and abilities.” Other attendees commented that deep dives into NBS processing helped them get a better understanding of how NBS works with ELR and eCR data.
The sessions were especially useful for staff working in new roles. “As someone walking in on week 3, day 1, in a new role, it was helpful getting such an in-depth introduction to the system and all of its functionality,” said one attendee. “It gave me suggestion points to take back for the team members to approach operability issues I’ve seen as a fresh set of eyes.”
To access materials and recordings from the training event, email nbs@cdc.gov.
Modernization virtual consultancy
In addition to the virtual training, CDC’s NBS modernization team held its first virtual consultancy on January 25, 2023. This event gave NBS users an opportunity to share their input on features and priorities for modernization. The consultancy helped CDC’s growing NBS team broaden collaboration with jurisdictions and is just one of the many ways CDC is receiving input from NBS users.
CDC presentations—including opening remarks from Dr. Jennifer Adjemian, director, CDC Division of Health Informatics and Surveillance—offered an overview of:
- how CDC uses human-centered design principles and user research to understand the needs of NBS users and
- how CDC uses these insights to prioritize new features and improvements.
After the presentations, participants joined break-out rooms for two interactive activities. The first activity asked participants to imagine a completely new disease surveillance system and brainstorm features and capabilities they would want in the system. The team encouraged participants to think about how they would build an ideal disease surveillance system without being constrained by currently available resources.
The second activity asked participants to share their thoughts about what works well in NBS today, what needs improvement, and what new opportunities they see. Insights from both activities will help CDC prioritize short-term next steps to modernize the current NBS while influencing a long-term vision for the future of NBS.
The team conducted polls at the beginning and the end of the virtual consultancy to measure the event’s effectiveness and inform planning for future events. Although more work is still ahead, the results show significant improvements in:
- trust that CDC is focusing NBS modernization on the needs of users and
- understanding of how CDC determines priorities for NBS modernization.
The team plans to present findings to jurisdictions on an upcoming weekly NBS Subject Matter Expert call.
To access the slides and recordings from the virtual consultancy event, email nbs@cdc.gov.
Change to display of NNDSS weekly tables on CDC WONDER
On January 17, 2023, the National Notifiable Diseases Surveillance System (NNDSS) released a new table format for NNDSS weekly data on CDC’s Wide-ranging Online Data for Epidemiologic Research (WONDER)!
A key data source for public health, the NNDSS weekly tables provide information on cases of selected infectious national notifiable diseases and conditions sent to CDC by U.S. states and territories. The changes are part of CDC’s efforts to modernize public health data systems.
In this new format, each condition is presented in a separate table. The new format will:
- allow users of NNDSS weekly data to focus on the condition of interest;
- enhance user experience compared to the previous, mixed condition(s) data tables; and
- make the NNDSS weekly data, text, and PDF files easier to use.
We invite you to explore these exciting new features! If you need additional information or have questions, please contact the CDC Electronic Data Exchange mailbox at edx@cdc.gov with the subject “NNDSS Weekly WONDER Tables.”
Have questions about case surveillance modernization?
Visit our Frequently Asked Questions page to find answers to common questions from state, local, and territorial jurisdictions.
Access archived NNDSS eSHARE webinars for monthly updates on case surveillance modernization.
Use this jurisdiction feedback form to provide feedback and ask questions.
The next eSHARE webinar, “Case Surveillance Data Standardization— Paving the Way for Increased Interoperability,” will be held February 21, 2023, 3:00–4:00 PM ET.
To join an eSHARE webinar, please see your calendar invitation or contact edx@cdc.gov for login information with the subject line “eSHARE invitation.”
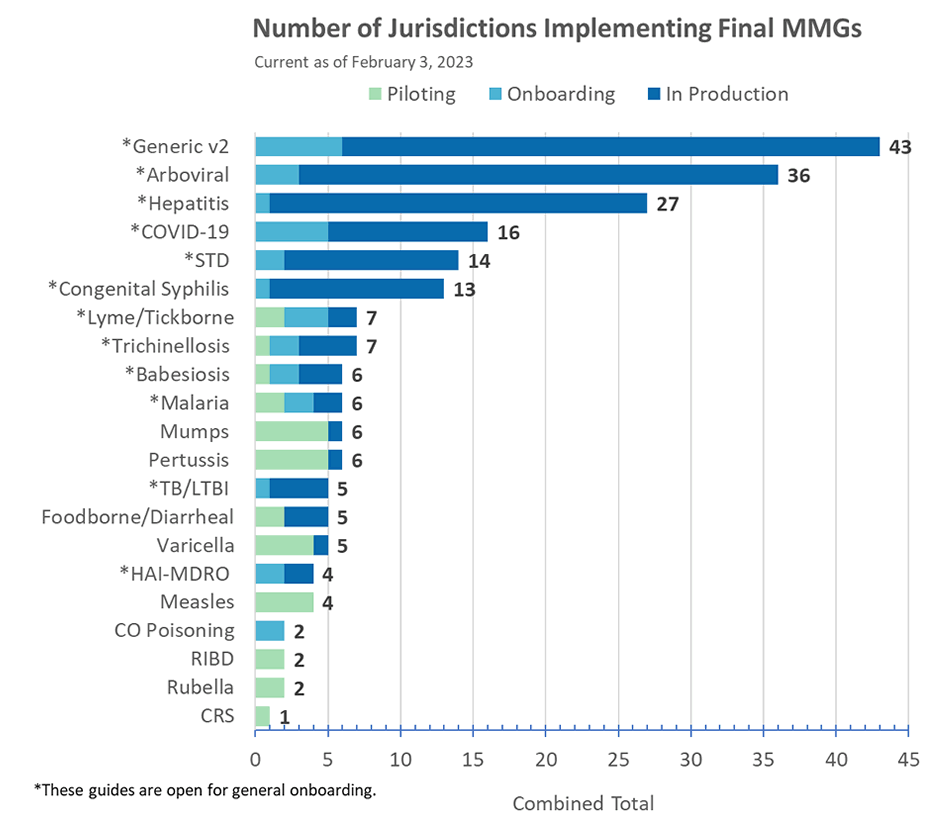
CDC is pausing most disease-specific message mapping guide (MMG) development and onboarding efforts. Learn more about how CDC is modernizing case surveillance. Get more information about CDC’s progress in this month’s News You Can Use section below.
Lyme and TBRD MMG
The PHIN Vocabulary Access and Distribution System (VADS) Lyme and Tickborne Rickettsial Diseases (TBRD) Case Notification View version 4 is now available for the Lyme and TBRD MMG. The changes in version 4 include:
- 15 additional values for Lab Test Type (Lyme) and
- four additional values for Lab Test Type (TBRD).
Congratulations to Kentucky for being in production for the Arboviral v1.3 MMG!
Case Surveillance Modernization Update
To enhance case surveillance and reduce burden on state, local, and territorial jurisdictions participating in the National Notifiable Diseases Surveillance System (NNDSS), CDC is collaborating with jurisdictions and public health partners to modernize case surveillance.
CDC is sharing recurring, operational progress updates through channels such as Case Surveillance News and monthly eSHARE training webinars. CDC will also share time-sensitive information about actions for jurisdictions, key milestones, and project decisions using the NNDSS email list via edx@cdc.gov.
Key implementation updates for February 2023:
- CDC is using existing Epidemiology and Laboratory Capacity (ELC) Cooperative Agreement Health Information Systems team calls with jurisdictions to verify status of MMG implementation. This information helps CDC provide customized guidance to jurisdictions who choose to move forward with implementing an MMG that aligns with current guidance.
- In collaboration with MITRE, the landscape assessment to define barriers and inform solutions for sending GenV2 core data elements is expected to begin in March 2023.
Jurisdiction support is critical to the success of this effort. CDC is continuing to work with jurisdictions to better understand their needs and status of MMG onboarding work. CDC encourages jurisdictions to give your input and feedback on any barriers or obstacles your organization may face with implementing the recommendations.
Visit CDC’s Frequently Asked Questions webpage to find answers to common questions from state, local, and territorial jurisdictions. Although we’re still working on the answers to many of your questions, CDC will continue updating this page as we have more information to share.
You can also use this jurisdiction feedback form to provide feedback and ask questions. We look forward to hearing from you!