October 2021
October 19, 3:00–4:00 PM ET: eSHARE webinar: What States Need to Know — Implementing the New NNDSS Malaria, Babesiosis, and Trichinellosis HL7 Case Notification Messages
November 15, 2021: Updated deadline to
- sign off on final 2020 reconciliation data and
- send NNDSS final, aggregate 2020 coronavirus disease 2019 (COVID-19) case counts
June 19–23, 2022: Council of State and Territorial Epidemiologists (CSTE) Annual Conference, Louisville, Kentucky. Learn more

Updated Timelines for 2020 NNDSS Reconciliation and Final, Aggregate 2020 COVID-19 Case Counts
The CDC NNDSS team is extending the deadlines for jurisdictions to complete 2020 NNDSS reconciliation procedures and send NNDSS final, aggregate 2020 COVID-19 case counts.
Wednesday, October 20, 2021, is the last day for jurisdictions to:
- request that the NNDSS Data Management and Reporting specialist “lock” their STD and non-STD data as final and
- confirm or update 2020 reporting exceptions.
Monday, November 15, 2021, is the last day for State/Territorial Epidemiologists to email final signature reports back to CDC.
Jurisdictions should plan to send their final, aggregate 2020 COVID-19 case counts to CDC by Monday, November 15, 2021, which is the same deadline for submitting final 2020 data for other NNDSS conditions.
For more information on the new deadlines, visit our archive of the September 21, 2021, eSHARE webinar.
Improvements to Message Mapping Guide Onboarding Process
The CDC Data Standardization and Assistance Team (DSAT) is proposing data-driven improvements to the onboarding process for HL7 message mapping guides (MMGs). To minimize jurisdiction resources needed to maintain legacy NNDSS formats during onboarding, ensure that there is no data loss for CDC programs, and reduce onboarding time, these improvements clearly define:

- roles and responsibilities,
- shared goals and expectations, and
- consistent and efficient timelines.
DSAT is currently gathering feedback from jurisdictions, CDC programs, and other key partners on the proposed process improvements. Once finalized, DSAT expects to implement the updated process in early 2022.
To learn more about proposed improvements to MMG onboarding, visit our archive of the September 21, 2021, eSHARE webinar. If you have feedback on the proposed changes, email edx@cdc.gov with the subject line “Updated Onboarding Process” by October 22, 2021.
October Topic: What States Need to Know — Implementing the New NNDSS Malaria, Babesiosis, and Trichinellosis HL7 Case Notification Messages
Mark your calendar for the next NNDSS eSHARE session, scheduled for Tuesday, October 19, 2021, from 3:00–4:00 PM ET.
To join a webinar, please see your Outlook invitation or contact the CDC Electronic Data Exchange mailbox at edx@cdc.gov for login information.
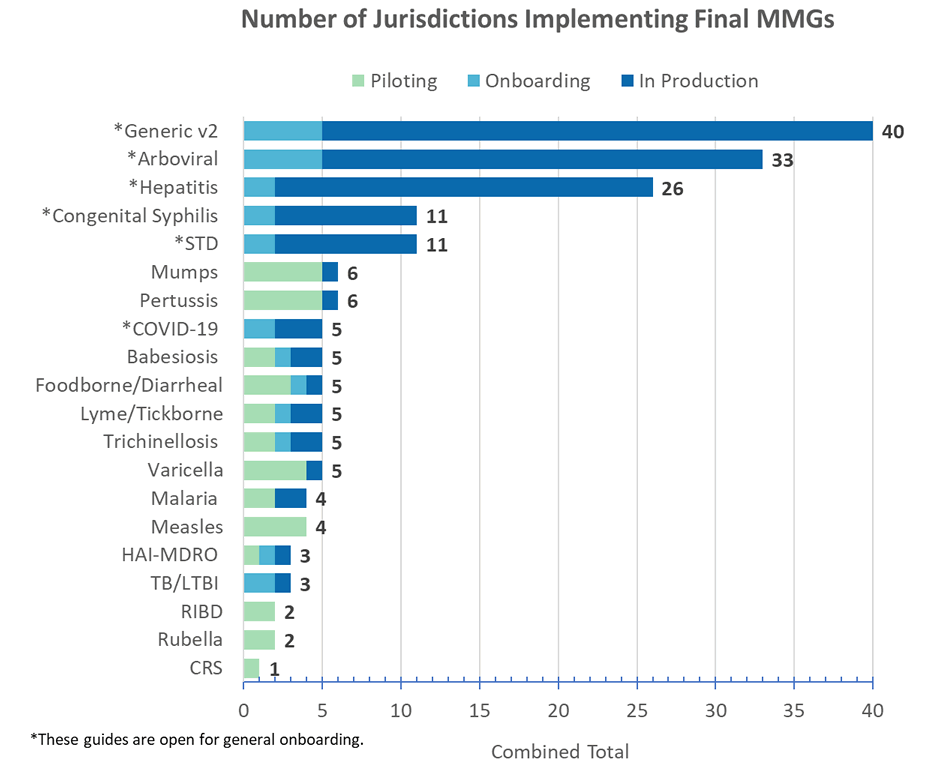
Babesiosis Message Mapping Guide
- CDC expects to open babesiosis for general onboarding by the end of October. Babesiosis MMG version 1.0.2 contains formatting updates and includes the most up-to-date value set for International Destination(s) of Recent Travel (82764-2).
COVID-19 Message Mapping Guide
- Version 1.1 of the COVID-19 MMG has now posted. This version adds three new data elements: two for reinfection and one in the laboratory repeating group to capture sequencing ID. All value sets associated with this guide are in the PHIN Vocabulary Access and Distribution System (VADS) COVID-19 Case Notification View version 9.
- The PHIN VADS COVID-19 Case Notification View version 10 is now available for COVID-19 MMG version 1.0 and v1.1, including four additional values to Lab Test Type (COVID-19) and three additional values for Vaccine Type (NND).
Malaria Message Mapping Guide
- CDC expects to open malaria for general onboarding by the end of October. Malaria MMG version 1.0.2 contains formatting and test scenario updates and includes the most up-to-date value sets for International Destination(s) of Recent Travel (82764-2) and Subject’s Country of Residence Prior to Most Recent Travel (TRAVEL15).
Trichinellosis Message Mapping Guide
- CDC expects to open trichinellosis for general onboarding by the end of October. Trichinellosis MMG version 1.0.3 contains formatting and test scenario updates and includes the most up-to-date value set for International Destination(s) of Recent Travel (82764-2).
If you are interested in joining a cohort or onboarding an MMG, please email the CDC Electronic Data Exchange mailbox at edx@cdc.gov.
Reminder for Jurisdictions about the Low-incidence Case Verification Process
Effective August 14, 2021, jurisdictions and CDC programs should use the Message Validation, Processing, and Provisioning System (MVPS) portal to verify low-incidence cases:
- When CDC receives a notification that meets the criteria for low-incidence case verification, MVPS will automatically email the jurisdiction requesting that you log into the MVPS portal to verify the case. The email from MVPS will include the CASE ID, condition name, and event code.
- Use the information in the email to search for the case in the low-incidence window and select a verification response.
- Verification responses include “under investigation,” “do not publish,” and “approved” for publication.
- MVPS will send weekly reminders for two weeks about any cases that need verification. If you have not entered a response after two weeks, NNDSS staff will contact you to request that you enter a low-incidence case verification response.
To learn more about the low-incidence case verification process, visit our archive of the August 10, 2021, eSHARE webinar for:
- a presentation providing an overview of the low-incidence case verification process (see slides 74-86) and
- a job aid for jurisdictions with instructions for how to use the MVPS portal to verify low-incidence cases. Learn how to get access to job aids on MVPS.
New Report Makes Recommendations for Modernizing Public Health Data Systems
As part of the Data Modernization Initiative, CDC encourages jurisdictions to re-think and re-design their information systems using the funding provided through the Health Information Systems component of the Epidemiology and Laboratory Capacity Cooperative Agreement.
As previewed in the May issue of Case Surveillance News, NNDSS asked the Public Health Informatics Institute (PHII) to develop recommendations for jurisdiction public health systems, recognizing that the original National Electronic Disease Surveillance System standards from 2001 are 20 years old. NNDSS asked that PHII engage stakeholders and experts, consider a time horizon of 5–10 years, and focus on functionalities and capabilities.
PHII developed recommendations using information gathered through listening sessions and validated them with an expert panel. The PHII report points out the importance of addressing the organizational, interorganizational, and workforce aspects of modernization, as well as technical aspects. The report also addresses the importance of achieving balance between jurisdiction needs and national harmonization.
These recommendations will be integrated with the CDC Data Modernization Initiative’s emerging data ecosystem vision and other data system and surveillance recommendations to produce one set of coordinated guidance for surveillance systems.
Read PHII’s recommendations at Data Modernization Initiative: Case-based surveillance capabilities and technology recommendations.
Highlights of PHII’s recommendations may be useful in your current planning:
- Establish automation through integration—Separate systems need to become more integrated and interoperable to achieve efficiencies. This will support a better and more timely flow of data and leverage investments by building a service once and using for multiple health department functions.
- Balance the push and pull—Develop the ability to pull data from electronic health records or other public health systems rather than waiting on a push of information. This may make data timelier, is likely to allow better control over data flow and processing, and may reduce the reporting burden for healthcare.
- Use artificial intelligence (AI)—AI might help public health handle large volumes of data more effectively and could support predictive analytics to enable public health to be more proactive. PHII acknowledges that while AI offers opportunities, other foundational improvements are more pressing.
- Establish uniform system standards and functionality—More consistency among systems would help support integration. This should include standardized functional requirements, technical requirements, and ways of collecting data across programs. Uniform system standards and functionality would make it easier for healthcare to interact with public health and for jurisdictions and jurisdiction programs to share data and resources. The recommendations emphasized centralized services, such as Reportable Conditions Knowledge Management System (RCKMS), and adoption of standards, such as Fast Healthcare Interoperability Resources (FHIR).
- Harmonize data across public health programs—Improve semantic interoperability and use common data standards such as United States Core Data for Interoperability (USCDI).
- Ensure flexibility and modularity of systems—To be more flexible, think of functions instead of systems, use modular design, and increase interoperability using application programming interfaces.
- Support scalable systems—Systems should be readily scalable to meet demands. Use of cloud services is important for achieving this.
- Improve entity and patient matching—Current approaches to matching records on individuals are a time drain and a hurdle to integration. Use agency-level master patient indexes supported by record locator services and identify cross-jurisdiction solutions.
- Improve partnerships and cross jurisdictional coordination—Relationships are important. The ability to share data across jurisdiction borders needs to be a fundamental requirement. Trusted sharing frameworks such as Trusted Exchange Framework and Common Agreement (TEFCA) will be important.
- Support intensive data access required for dedicated query systems—Use data lakes and data warehouses to improve access to and use of data.
- Streamline or simplify data transport—Get as much data through one pipe as possible and standardize expectations. This would help healthcare and create efficiencies for public health.