NIOSH-Approved Particulate Filtering Facepiece Respirators
This site provides a listing of NIOSH-approved particulate filtering facepiece respirators. This type of air-purifying respirators protects by filtering particles out of the air the user is breathing. There are seven classes of filters for NIOSH-approved filtering facepiece respirators available at this time. Ninety-five percent is the minimal level of filtration that will be approved by NIOSH. The N, R, and P designations refer to the filter’s oil resistance as described below.
N95 – Filters at least 95% of airborne particles. Not resistant to oil.
(N95 Manufacturers Index: 3M A B C D E F G H I J K L M N O P Q R S T U V W X Y Z)
N99 – Filters at least 99% of airborne particles. Not resistant to oil.
N100 – Filters at least 99.97% of airborne particles. Not resistant to oil.
R95 – Filters at least 95% of airborne particles. Somewhat resistant to oil.
P95 – Filters at least 95% of airborne particles. Strongly resistant to oil.
P99 – Filters at least 99% of airborne particles. Strongly resistant to oil.
P100 – Filters at least 99.97% of airborne particles. Strongly resistant to oil.
The NIOSH-approved products are listed by brand. Links to the manufacturers’ websites are provided as a courtesy to users and NIOSH is not responsible for the content of those pages. Also included is the manufacturer’s phone number, product model number, approval number (84A-XXXX), an indication if the product has an exhalation valve, and the user donning instructions. The manufacturer’s donning procedure and/or user instructions are also provided here as a courtesy to the user.
Manufacturers’ recommended procedures for performing a user seal check can be included in the donning procedures and/or user instructions as alternatives to the OSHA-specified procedures under the respiratory protection standard (See 29 CFR 1910.134 Appendix B-1external icon). NIOSH does not evaluate the efficacy and reliability of any user seal check procedures, but OSHA will accept the manufacturer’s recommended procedures if the employer demonstrates those procedures are equally effective as those identified in the standard.
Each manufacturer is responsible for updating the links on their website and/or providing NIOSH with an updated or revised copy when changes are made.
The tables were created to provide easy access to a comprehensive listing of NIOSH-approved particulate filtering facepiece respirators and also to provide easy access to the donning process/user instructions. The tables are not updated as frequently as the certified equipment list, which is the official NIOSH certification record.
Searching for a Product Using the Certified Equipment List
If you have a product that is not listed on the provided tables use the searchable certified equipment list.
Follow these steps to search for NIOSH-approved disposable particulate respirators:
- In For Protections Against section, select N95, N99, N100, R95, P95, or P100.
- In Facepiece Type section, select only Filtering Facepiece.
- Select View Results.
If your product is not listed, you should scroll through the list of “Private Label” products.
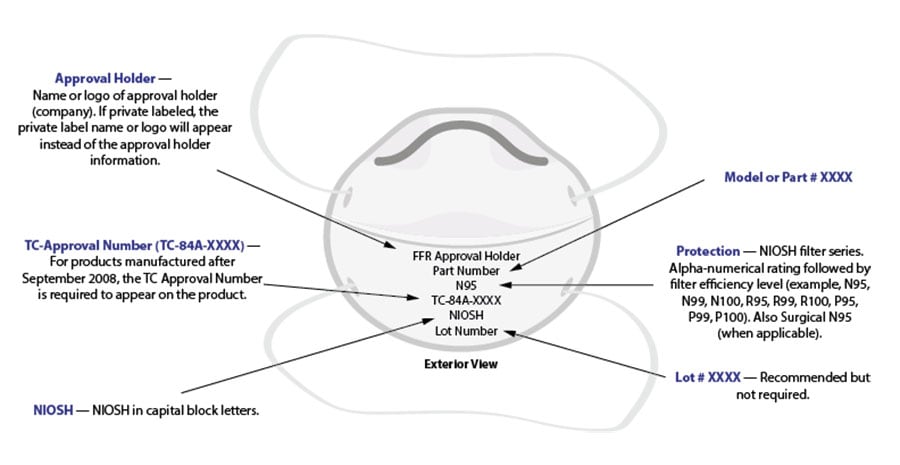
Filtering Facepiece Respirator (FFR) Labels
Individual filtering facepiece respirators are required to have the following markings:
- Name of approval holder/manufacturer business name, a registered trademark, or an easily understood abbreviation of the applicant/approval holder’s business name as recognized by NIOSH. When applicable, the name of the entity to which the FFR has been private labeled by the approval holder may replace the approval holder business name, registered trademark, or abbreviation of the approval holder business name as recognized by NIOSH.
- NIOSH in block letters or the NIOSH logo.
- NIOSH Testing and Certification approval number, e.g., TC-84A-XXXX.
- NIOSH filter series and filter efficiency level, e.g., N95, N99, N100, R95, P95, P99, P100.
- Model number or part number: The approval holder’s respirator model number or part number, represented by a series of numbers or alphanumeric markings, e.g., 8577 or 8577A.
NIOSH recommends the lot number and/or date of manufacture also be included, however, this is not required.
Sample of a generic filtering facepiece respirator with appropriate markings.
Filtering facepiece respirators that are private labeled are required to have the following statement on the packaging as a special S caution and limitation statement identified on the full label and located in the respirator user instructions:
- Marketed by xxxxxx (the private label company name).
- Produced by xxxxxx (the approval holder company name).
This private label related statement does not need to appear on the exterior surface of the respirator as part of the required name marking.
