Pilot Plant Operator Killed in Pressure Vessel Release at a Massachusetts Biotechnology Company
Massachusetts Case Report: 94MA019
December 20, 1994
Summary
On May 24, 1994 a 22 year old male pilot plant operator was fatally injured at a Massachusetts biotechnology company when he opened a pressure vessel which was still under pressure. The victim was extracting the compound taxol from yew needles in a near “super critical” fluid process which employed methanol and carbon dioxide, at a pressure of 2,000 pounds per square inch (psi). The victim, who was alone, was at the end of the procedure when the pressure release occurred. Apparently believing the vessel to be depressurized, the victim attempted to remove the heavy steel cover. It appears he removed the four bolts holding the cover in place, and was attempting to remove the retaining flanges when the cover of the vessel blew. The pressure was released, throwing the victim 10 feet across the room. An assistant to the company president noted that the building’s alarm and sprinkler systems had been activated, and alerted the president as he was leaving the building. Within 15 seconds the president found the victim, with a slight pulse, entangled in equipment. Paramedics were immediately summoned. The victim was transported to a hospital where he was pronounced dead later than night. In order to prevent future similar occurrences, the MA FACE Program recommends that employers:
- and equipment manufacturers install safe guards, such as mechanical interlocks, on pressure vessels to ensure that they are completely depressurized prior to being opened
- use a system of “checks and balances” to verify whether residual pressure remains in pressure vessels
- ensure that all operators of pressure vessels are fully trained prior to allowing them to operate a system
- use procedures for minimizing the potential for materials blockage in those supercritical extraction processes prone to blockage
- develop procedures to insure the purity grade of solvents used in supercritical fluid systems
- ensure that process equipment and operations undergo safety reviews before going into service in research, pilot or production capacities.
In addition, government agencies should:
- consider developing safety standards to protect employees who operate pressure vessels.
Introduction
On May 25, 1994, the Medical Examiners Office notified the MA FACE Project through its 24 hour fatality hotline that a 22 year old male pilot plant operator had been killed in an explosion at a biotechnology firm the previous evening. An investigation was immediately initiated.
The MA FACE Project Director visited the incident site on May 27 and June 1, and interviewed several of the victim’s coworkers, the victim’s supervisor, and the president of the company. The investigating fire marshall, Occupational Safety and Health Administration (OSHA) compliance officer, and several experts knowledgeable in supercritical fluid processes were also consulted during the investigation.
The death certificate, police, and OSHA reports were obtained during the investigation. Photographs were taken of the incident site. A schematic of the system, the written protocol, and the victim’s log notes on the run were collected. A consulting firm report, documenting the physical condition of the system, was also obtained.
The company was a research and development, biotechnology company specializing in anti-viral and anti-carcinogen biotherapeutics. Approximately 60-70% of the company’s operations involved near supercritical fluid processes, using highly pressurized systems. The supercritical fluid process uses a combination of high pressure and temperature conditions in order to transform carbon dioxide (CO2) into a “supercritical,” or highly densified gas, phase. In the so-called supercritical phase, carbon dioxide acts as an excellent solvent with multiple uses.
The company had been in business for 6 years, and employed 21 people. There were two full time and two part time pilot plant operators. The company did not have a written safety program, although it did provide basic safety training for all new employees, including such topics as fire safety and hazard communication. The company employed a designated safety officer who devoted less than 25% of her time to safety. While there was no safety committee, safety issues were often addressed during the company’s weekly general meetings.
Pilot plant operators were not given specific training about the hazards of high pressure systems. Certain operations of the equipment required relying on subjective clues; these were not taught, but gained through experience. The phone numbers of supervisors and coworkers were routinely made available for the pilot plant operators running the equipment at night on their own.
The victim had been employed for approximately 2 weeks as an intern; he was a chemical engineering student. The victim received a week of training on the taxol extraction system, and had been previously employed by a company which used supercritical fluid systems. Although he had run the extraction procedure six times on his own, the victim had never before performed the run in the evening without immediate supervision.
Investigation
Background on the system:
The company extracted the compound taxol from yew needles in a supercritical fluid system designed and built by the employer. The extraction was conducted in a 5.2 inch diameter, 6 foot tall, 25 liter, cylindrical steel vessel rated for 5,000 psi (the “extractor”). Chromatography columns were also used to aid in the separation of the compounds. The entire system was enclosed in a plexiglass room approximately 10 feet tall and 6 feet long.
Approximately 75-90% carbon dioxide and 10-25% methanol were used. Carbon dioxide was introduced into the system from liquid carbon dioxide “gas” cylinders pressurized at 800 psi. A compressed air driven pump pressurized the system to 2,000 psi. A back pressure regulator was used to maintain the extractor pressure at 2,000 psi. At the time of the incident, the company had discontinued the use of direct heat to facilitate the transformation of carbon dioxide to the supercritical phase.
Two pressure gauges were used to monitor the pressure inside the vessel. One was placed at the inlet line and the other at the outlet line of the extractor. Both displayed pressures up to 6,000 psi and were graduated at intervals of 1,000 psi. The inlet pressure gauge was located upstream from a ball valve and an inline porous metal filter. The outlet pressure gauge was located downstream from the top of the vessel, which contained a sintered porous metal filter, and from a series of ball valves. (see diagram for simplified schematic of the system)
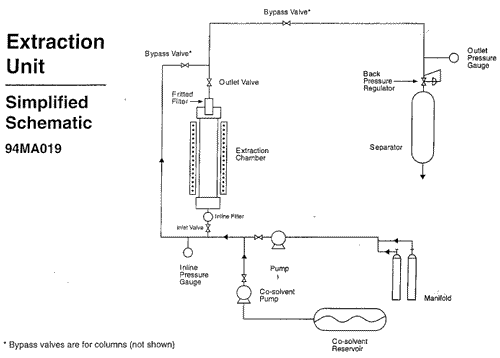
The standard operating procedure was to first wash a load of yew needles with carbon dioxide at 2,000 psi. Methanol was then pumped into the system at two different flow rates. The first flow rate was slower, and allowed for the methanol to mix completely with the carbon dioxide. Methanol was then pumped in at a higher rate. At the higher rate the methanol would not mix with the CO2, but would instead form a “plug” consisting of a highly compact mixture of methanol and yew material. The plug was intentionally formed in order to collect an identifiable fraction of the yew plant containing the non-polar waxes. Fractions from the plant were collected from the separator throughout the extraction.
After the plug was formed, the system was again washed with CO2 in order to purge the methanol. The final CO2 wash completed the extraction, and at this point depressurization would begin. Depressurization was achieved by opening a system of valves at the top (outlet) and the bottom (inlet) of the extractor. Although the written protocol called for draining the extractor from both the top and bottom of the vessel, depressurization could be achieved by opening only one end of the extractor (see diagram ). Carbon dioxide exited the system through a separator, where it was collected in the form of dry ice. The dry ice sublimed to carbon dioxide gas and was vented from the plexiglass enclosure to the outside. Under normal circumstances, depressurization took 1 hour. After depressurization, the top closure of the vessel was removed and the yew needles were removed from the chamber.
The vessel was sealed at both ends with metal closures. Each weighed 60 lbs, and was held in place by 2 thick metal flanges. The flanges weighed 40 lbs each and were held together with 4 bolts. A porous metal filter, 3/8 inch in diameter and 6 inches long, fit inside the top cover.
There was no standard operating procedure for removing the top closure, although the routine generally followed by the pilot plant operators was as follows. Two caps (1/4 inch and 3/8 inch) on the vessel closure were first removed in order to check for residual pressure. The 3/8 in. cap covered the opening for the metal filter and the 1/4 in. opening was for a thermometer, no longer used. If the operator could not hold his/her finger over the 3/8 inch opening due to the release of pressure, then the chamber was considered pressurized and given more time to drain. A loud hissing noise would indicate to the operators that the system still contained substantial pressure. If the caps could be removed without substantial pressure release, the operator would proceed to remove the top of the vessel.
In order to remove the closure from the extractor, the operator stood on a ladder. The bolts were unscrewed and removed, and then the flanges were removed. Due to their heaviness, it was necessary to knock the flanges with one of the bolts, or some other heavy object, before removing them. The operators routinely leaned into the flanges to get the leverage required to move them. Once the flanges were removed, the closure was lifted from the extractor.
The Incident:
The company had decided to run a series of experiments aimed at reducing the run time and the amount of methanol used. The pilot plant operators and their supervisor had outlined a plan for six experimental runs. On the day of the incident, the victim chose to run one of the experiments which called for using the entire volume of methanol at the higher of the two flow rates. In addition to the higher flow rate of methanol, the chromatography columns were not employed. Furthermore, the victim used recovered methanol. It was the only methanol available on the day of the incident.
The victim started his run at 4:00 PM, and by 5:22 PM he had completed the first carbon dioxide wash, pumped the methanol into the system, and collected the first two fractions of the extract. At 5:45 PM he noted in his log that the reactor had become plugged, and that CO2 flow had stopped. At this time the victim recorded an outlet pressure reading of 800 psi. Just 17 minutes earlier the victim had recorded an outlet pressure reading of 2,000 psi. According to coworkers, a rapid pressure drop such as this is indicative of a plug in the extractor; normally the pressure is released very slowly. Alternatively, the victim may have intentionally opened the back pressure regulator in order to lower the pressure and increase flow through the system. In so doing, he may have unintentionally created a plug in the extractor.
At 6:01 PM, the victim again noted in his log that the CO2 flow was very slow, and at 6:15 he decided to depressurize, noting that the plug of methanol was not being pushed through the reaction chamber. In the victim’s next entry, however, at 6:36 PM, he noted that he was re pressurizing the system (presumably to purge the methanol from the system). At this time he recorded an inlet pressure of 2400 psi, and an outlet pressure of 1700 psi. According to coworkers, this pressure differential is another indicator of a plugged reactor.
Finally, at 7:07, the victim recorded in his log “not working, total depress.” He then waited at least an hour and a half for the system to drain. The incident occurred at about 8:45 PM.
An assistant to the company president was alerted to the incident when he noted that the building’s alarm and sprinkler systems had been activated. He immediately notified the president who was leaving for the evening and was outside of the building. The president rushed to the pilot plant and within 15 seconds found the victim, with a slight pulse, entangled in some equipment approximately 10 feet away from the reaction chamber. Paramedics were immediately summoned, and the victim was transported to a hospital.
When the emergency responders found the victim, one of the flanges was laying next to him. The vessel cover and the other flange were found close to the plexiglass room. The cover apparently shot straight up 12 feet, hitting and indenting the roof, and then fell to the floor close to the extractor. Two of the bolts were found on top of the plexiglass box, and two were found on the floor by the extractor. None of the 4 bolts were stripped or otherwise damaged, indicating that the victim had removed them before the pressure was released. Similarly, the fitting on the closure was not damaged, indicating that the victim had removed it before the incident. The 1/4 inch cap was also found on top of the plexiglass box.
The victim apparently drained the system from only the bottom of the chamber. The first investigators on the scene observed that the valves for draining from the bottom were all opened while the top valve was closed. Apparently the victim made a decision to close the top valve; coworkers reported that the valve must be open during normal operation. All of the other valves were found properly positioned.
The incident does not appear to have resulted from a faulty pressure gauge or valve. The company hired an independent engineering consulting firm to disassemble the extraction unit and document the conditions of gauges, filters, valves and piping components. The firm concluded that three of the ball valves, the back pressure regulator, and the pressure gauges were found to be intact and in proper working order. The filters were found to be functional but the report did not state whether they were clear of material.
Possible Scenarios to Explain the Incident:
FACE concluded that the vessel had a blockage, and that there were several possible ways in which the vessel could have become blocked. Regardless of the nature of the blockage, however, most individuals involved in the case concur that the amount of pressure released was most likely low (less than 100 psi). The pressure is thought to be low based on approximate force calculations. Given the surface area and weight of the cover, a pressure release of much greater than 100 would have blown the cover through the roof.
The vessel could have become blocked in the following ways. First, plugs of material may have formed in either or both of the filters at the bottom and top of the vessel. Second, the inlet line may have frozen during depressurization, and thus caused a blockage in the system. Finally, a blockage may have been created by a plug of methanol laden yew material within the vessel.
Plugs were reportedly a common phenomenon in the system. Coworkers noted that blockages had occurred previously in the chromatography columns. Similarly, it was reported that material was often so compact in the top portion of the vessel after a run that the material had to be removed with a metal scoop. Due to the tendency for the top (outlet) of the vessel to become densely compacted, it is likely that material may have clogged the metal filter in the cover. The outlet pressure gauge would not have indicated such a blockage because it was located on piping downstream from the closure and several ball valves. Moreover, the outlet valve which was located between the closure and the pressure gauge was found closed. A blockage in the cover of the vessel could also explain why the victim may not have detected much residual pressure release when he unscrewed the top fitting and thermometer cap.
Although there was not a similar history of blockage in the bottom of the extractor, the inlet filter may also have been clogged. If this filter had been blocked, the inlet pressure gauge would not have indicated that the system was still pressurized because the gauge was located between the extractor and the filter.
The second scenario, that the inlet line froze during depressurization, is perhaps the least likely due to the visual clue of frosting which the victim may have noted. It was, however, a common occurrence for the lines and the separator to ice up during decompression, due to the endothermic effect of the CO2 pressure drop. Icing of the line may have occurred in combination with a material plug at the top of the chamber.
In the third scenario, a plug of methanol laden yew material may have formed within the vessel, creating a sharp concentration gradient between the supercritical carbon dioxide and the methanol. Such a plug could have frozen when the temperature dropped during depressurization, and remained frozen while the CO2 drained from the extractor. Unless the pressure gauges were located on the vessel itself, a pressure build up resulting from a concentration gradient would not have been detected.
The possibility that a plug formed within the extractor is likely for the following reasons. First, OSHA determined that the recovered methanol contained almost 50% water (the contents were 50.9% methanol, trace organics, and water). The high percentage of water in the methanol could have contributed to the formation of plugs in the extractor. At a minimum, the water would have lowered the freezing point of the methanol. However, hydrates (CO2 and water compounds with a freezing point of 50o F) may also have formed. Second, it is possible that the methanol and CO2 may have gone into a phase where they were no longer miscible because a relatively high flow rate of methanol was used in the experimental run.
Finally, it is important to consider that the pressure gauges may not have indicated that there was pressure in the system simply because their accuracy was limited at low pressures. The pressure gauges were graduated by 1,000 psi, and their margin of error was 10 percent. If the pressure remaining in the system was less than 100 psi, it is possible that the gauges may have indicated that the pressure was approximately zero.
Cause of Death
The medical examiner listed the cause of death as multiple injuries.
Recommendations/Discussion
Recommendation #1: Employers and equipment manufacturers should equip pressure vessels with safe guards, such as mechanical interlocks, in order to ensure that the vessels have been completely depressurized prior to being opened.
Discussion: The extraction unit’s two pressure gauges did not indicate that pressure remained in the extractor. This may have occurred due to the location of the gauges (on piping connected to either end of the vessel), or because the gauges could not indicate low pressures with accuracy. Employers and equipment manufacturers should install pressure gauges or pressure transducers directly on pressure vessels, rather than on piping connected to the vessel. Furthermore, employers should utilize pressure gauges which are capable of accurately registering both high and low pressures (e.g. a pressure manifold). In addition, employers should install pressure release valves on extractors. Flush diaphragm transducers minimize the potential for materials blockage in the lines leading to the pressure indicator. Had pressure gauges been welded to the top and the bottom of the vessel, the victim may have detected the residual pressure in the reactor, and this incident may have been averted.
Even when pressure gauges are located directly on pressure vessels, however, it is still possible for blockages to occur but not be detected. This situation could arise if the blockage were to occur in a localized area distant from the gauge. An effective means for guarding against this possibility would be an interlock system built into the closure. Employers and equipment manufacturers should develop and use covers for pressure vessels which could not be removed if pressure remained in the vessels. Use of such an interlock system would eliminate the need to rely solely on pressure gauges and subjective visual clues.
A large full port vent or drain mounted directly on the closure would also allow the operator to test for pressure behind the closure before removing it. The operator could open the valve and check for plugs using a “dip stick.”
Recommendation #2: Employers should use a system of “checks and balances” to verify whether residual pressure remains in pressure vessels.
Discussion: For optimal safety, employers should use several mechanisms to verify that pressure vessels do not contain residual pressure. Pressure gauge readings should never be the only means for determining whether a vessel has been depressurized. Ideally, an extractor should be equipped with an interlocking closure and pressure manifold. In addition, several procedures (such as using a dipstick to check for plugs or running a measurable quantity of inert gas through the system) should be employed to ascertain that there are no blockages in the system. Had a system of checks and balances been used, this incident may have been prevented.
Recommendation #3: Employers should ensure that all operators of supercritical fluids systems have had extensive training prior to allowing them to operate a system.
Discussion: The victim had received only one week of training on the extraction system before he was allowed to run it alone at night without immediate supervision, and successful operation of the system required a reliance on subjective clues which were not taught, but gained through experience. Employers should provide all operators of supercritical fluid systems with in depth training on how to run particular systems. The hazards of working with high pressure systems, and the subjective clues to look for when trouble shooting problems, should be included in this training. Furthermore, employers should ensure that employees understand that seemingly low levels of pressure (e.g. less than 100 psi) can be extremely hazardous and destructive if released under particular circumstances, such as over large surface areas. Employers should also consider requiring all operators of supercritical fluid systems to complete apprenticeships under close supervision prior to allowing them to operate systems on their own.
In addition, employers should develop written protocols which include detailed instructions for both routine operation and troubleshooting, and should consider allowing only senior operators or supervisors to trouble shoot. Finally, employers should consider requiring key decisions on operations, such as when a system is depressurized, to be made by two operators. If the victim had received more in depth training on the taxol system prior to being left alone to run it, or if two people had been required to make key decisions on operations, this incident may have been prevented.
Recommendation #4: Employers should use procedures to minimize the potential for materials blockage in those supercritical extraction procedures prone to blockage.
Discussion: The yew needles in this system had a tendency to form blockages, and impede the flow through the system. In supercritical fluid processes prone to blockage, employers should develop methods for minimizing the potential for materials blockage. For example, an inner mesh bag could be used inside of extractors to contain materials such as needles. A mesh bag could be used with a pore size which allowed for the free flow of solvents and extracted compounds, but restricted the diffusion of solid material.
Furthermore, when appropriate, heat should be used in supercritical fluids processes to minimize the tendency for vessel lines to freeze during decompression. Heating the system during decompression can facilitate the conversion of carbon dioxide from the supercritical to the gaseous phase, while reducing the tendency for the gas to sublime, or rapidly convert from the gaseous to the solid phase.
Recommendation #5: Employers should develop procedures for insuring the purity grade of solvents used in supercritical fluids systems.
Discussion: OSHA determined that the recovered methanol was comprised of almost 50% water. The relatively high percentage of water in the methanol may have affected the phases of the materials in the system. This in turn could have contributed to the blockage in the system. Employers should ensure the purity of the solvents they use in supercritical fluid extraction systems.
Recommendation #6: Employers should ensure that process equipment and operations undergo safety reviews before going into service in research, pilot or production capacities.
Discussion: In order to best anticipate any safety problems which might arise during operation of new or modified systems, employers should subject the systems to safety reviews. If employers do not have expertise in this area, they should seek the assistance of outside contractors.
Recommendation #7: Government agencies should develop safety standards to protect employees who operate pressure vessels.
Discussion: Other than enforcement of the American Society of Mechanical Engineers (ASME) Boiler and Pressure Vessel Code, there are no federal or state regulations concerning the safe operation of pressure vessels. The ASME “U” standard pertains to vessel design and construction, and is restricted to vessels with a diameter of at least 6 inches; however, this is not generally used as a requirement. The standard includes a provision requiring annual inspection. In this incident the vessel was 5.2 inches in diameter, and thus not covered by the ASME standard. Government agencies should develop safety standards to protect the employees who operate pressure vessels in manufacturing and pilot plant operations. In addition, government agencies should consider developing a certification process in which operators would be required to undertake specified training and pass an exam in order to obtain a license for operation.
Reference
American Society of Mechanical Engineers, Boiler and Pressure Vessel Code, Section VIII, 1992 and addendum.
To contact Massachusetts State FACE program personnel regarding State-based FACE reports, please use information listed on the Contact Sheet on the NIOSH FACE web site Please contact In-house FACE program personnel regarding In-house FACE reports and to gain assistance when State-FACE program personnel cannot be reached.
