Investigation of SARS-CoV-2 Transmission Associated With a Large Indoor Convention — New York City, November–December 2021
Weekly / February 18, 2022 / 71(7);243–248
Samira Sami, DrPH1; Libby Horter, MPH1,2; Diana Valencia, MS1; Isabel Thomas, MPH1,3; Mary Pomeroy, MSN1; Brianna Walker, MPH1; Sarah E. Smith-Jeffcoat, MPH1; Jacqueline E. Tate, PhD1; Hannah L. Kirking, MD1; Nang Thu Thu Kyaw, PhD4; Rebecca Burns, MPH4; Kathleen Blaney, MPH4; Vajeera Dorabawila, PhD5; Rebecca Hoen, DrPH5; Zachary Zirnhelt6; Cody Schardin6; Anna Uehara, PhD1; Adam C. Retchless, PhD1; Vance R. Brown, MA1; Yonathan Gebru, MPH1; Charles Powell, MS7; Stephen M. Bart, PhD7,8; Johanna Vostok, MPH9; Hannah Lund, MPH10,11; Jessica Kaess, MPH12; Megan Gumke, MPH13; Randy Propper, PhD13; Deepam Thomas, MPH14; Mojisola Ojo, MPH14; Alison Green, MPH15; Morgan Wieck, MPH15; Erica Wilson, MD16; Ryan J. Hollingshead, MA17; Sheila V. Nunez, MS17; Dawn M. Saady, MS18; Charsey Cole Porse, PhD19; Kyle Gardner, MSPH19; Daniel Drociuk20; Julia Scott, MSPH20; Taidy Perez, MPH20; Jim Collins, MPH21; Julie Shaffner, MS, MPH22,23; Ian Pray, PhD24; Laura T. Rust, MPH25; Shane Brady, MPH25; Janna L. Kerins, VMD26; Richard A. Teran, PhD8,26; Victoria Hughes27; Victoria Sepcic, MPH27; Eleanor W. Low, MS28; Sarah K. Kemble, MD28; Alexandra Berkley, MPH29; Kate Cleavinger, PhD29; Haytham Safi, MD30; Lindsey Martin Webb, MPH31; Scott Hutton, PhD32; Courtney Dewart, PhD23,33; Kristen Dickerson, PhD33; Eric Hawkins, MS34; Javeria Zafar, MPH35,36; Anna Krueger, MS37; Dena Bushman, MSN, MPH23,37; Bailee Ethridge, MS38; Katrina Hansen, MPH38; Jake Tant, MPH39; Christy Reed40; Carla Boutwell41; Jennifer Hanson41; Meagan Gillespie42; Matthew Donahue, MD43; Pilar Lane, DrPH43; Ruby Serrano, DrPH44; Lorena Hernandez, MS44; Michelle A. Dethloff45; Ruth Lynfield, MD6; Kathryn Como-Sabetti, MPH6; Emily Lutterloh, MD5; Joel Ackelsberg, MD4; Jessica N. Ricaldi, MD, PhD1 (View author affiliations)
View suggested citationSummary
What is already known about this topic?
The SARS-CoV-2 Delta (B.1.617.2) and Omicron (B.1.1.529) variants are highly transmissible. Outbreaks have been reported among vaccinated populations in indoor settings where mask use was limited.
What is added by this report?
Despite multiple introductions as evidenced by detection of at least three sublineages of SARS-CoV-2, this investigation did not find evidence of widespread transmission among a highly vaccinated population at a large event in an indoor setting where mask use was required and monitored.
What are the implications for public health practice?
Implementing multiple prevention measures (vaccinations and boosters, consistent mask wearing, enhanced indoor ventilation, and testing after text notification) can limit the transmission of SARS-CoV-2 at large events, including highly transmissible variants.
During November 19–21, 2021, an indoor convention (event) in New York City (NYC), was attended by approximately 53,000 persons from 52 U.S. jurisdictions and 30 foreign countries. In-person registration for the event began on November 18, 2021. The venue was equipped with high efficiency particulate air (HEPA) filtration, and attendees were required to wear a mask indoors and have documented receipt of at least 1 dose of a COVID-19 vaccine.* On December 2, 2021, the Minnesota Department of Health reported the first case of community-acquired COVID-19 in the United States caused by the SARS-CoV-2 B.1.1.529 (Omicron) variant in a person who had attended the event (1). CDC collaborated with state and local health departments to assess event-associated COVID-19 cases and potential exposures among U.S.-based attendees using data from COVID-19 surveillance systems and an anonymous online attendee survey. Among 34,541 attendees with available contact information, surveillance data identified test results for 4,560, including 119 (2.6%) persons from 16 jurisdictions with positive SARS-CoV-2 test results. Most (4,041 [95.2%]), survey respondents reported always wearing a mask while indoors at the event. Compared with test-negative respondents, test-positive respondents were more likely to report attending bars, karaoke, or nightclubs, and eating or drinking indoors near others for at least 15 minutes. Among 4,560 attendees who received testing, evidence of widespread transmission during the event was not identified. Genomic sequencing of 20 specimens identified the SARS-CoV-2 B.1.617.2 (Delta) variant (AY.25 and AY.103 sublineages) in 15 (75%) cases, and the Omicron variant (BA.1 sublineage) in five (25%) cases. These findings reinforce the importance of implementing multiple, simultaneous prevention measures, such as ensuring up-to-date vaccination, mask use, physical distancing, and improved ventilation in limiting SARS-CoV-2 transmission, during large, indoor events.†
An indoor convention in NYC with approximately 53,000 attendees was held during November 19–21, 2021. The facility was equipped with HEPA filters, and attendees were required to have documented receipt of at least 1 dose of COVID-19 vaccine and to use face masks while indoors. On December 2, 2021, the Minnesota Department of Health identified a case of COVID-19 caused by the Omicron variant in an attendee. State and local health departments collaborated with CDC to determine the extent of transmission during the convention and to make public health recommendations.
Two primary data sources were used in this investigation. The first was a list of attendees residing within the jurisdictions of participating state and local health departments. These attendees were matched with data from COVID-19 surveillance systems using personal identifiers (name and complete or partial address). Health departments identified positive and negative SARS-CoV-2 test results, demographic data, and vaccination histories§ for attendees. An event-associated case was defined as SARS-CoV-2 infection confirmed by reverse transcription–polymerase chain reaction or antigen testing in an event attendee during November 18–December 5, 2021. Sequencing of available specimens was conducted by state public health laboratories using multiple platforms¶; variant identification results were shared with CDC.
The second data source, an online anonymous survey, was administered via text message (29,766 text messages sent) and email (28,893 emails delivered) to approximately 35,000 attendees from 52 jurisdictions with available contact information, during December 11–19, 2021. Respondents were asked to report SARS-CoV-2 testing history and results, COVID-19 vaccination status, symptom history,** and exposure data during the event, and close contacts during and after the event. Available surveillance information and survey responses from U.S. resident attendees who received positive and negative test results were compared. Wilcoxon rank-sum tests were used for continuous data, and Pearson’s chi-square or Fisher’s exact tests were used for categorical data; statistical significance was defined as p<0.05.†† This activity was reviewed by CDC and was conducted consistent with applicable federal law and CDC policy.§§
Using COVID-19 surveillance systems, 48 public health jurisdictions reviewed data for 34,072 registered attendees; 39 jurisdictions reported a positive or negative result for 4,560 (13.4%) attendees, including 13 (<1%) self-tests¶¶ (from two states) (Table 1). Among 3,845 (84.3%) attendees with test and vaccination data, 3,248 (84.5%) had received a primary vaccination series, an additional 467 (12.1%) had received a booster dose,*** and 130 (3.4%) were partially vaccinated.
Among the 4,560 attendees with test result data, 119 (2.6%) event-associated cases were identified by January 6, 2022, from 16 jurisdictions (Figure). Among event-associated cases the median age was 26.5 years (IQR = 23.0–36.6 years), 65 (54.6%) were New York residents, and among 116 with gender data available, 54 (46.6%) were male (Table 1). Vaccination information was available for 88 persons with event-associated cases, 85 (96.6%) completed vaccination, including five who had received a booster dose. Among event-associated cases, the median interval from completing primary vaccination series to positive test result was 210 days (IQR = 193–232 days), and from booster dose to positive test result was 14 days (IQR = 12–20 days). Among the 3,630 (80%) test-negative attendees who completed primary vaccination or received booster dose, the median interval from completion of primary vaccination series to test date was 207 days (IQR = 187–225 days) and from receipt of booster dose to test date was 34 days (IQR = 22–66 days). One attendee with event-associated COVID-19 was hospitalized; no deaths were reported.
Genomic sequencing of 20 specimens identified the Delta variant (AY.25 and AY.103 sublineages) in 15 (75%) cases, and the Omicron variant (BA.1 sublineage) in five (25%). All attendees with Omicron cases were part of a known epidemiologic and phylogenetic cluster (2); no Delta variant cases were part of a cluster.†††
Among 7,259 respondents from the online survey (approximately 21% response rate) across 48 jurisdictions, 4,259 attendees reported receiving a COVID-19 test during November 18–December 5, 2021 (Table 2). Among these, 48 (1.1%) respondents from 10 jurisdictions reported SARS-CoV-2 infections during the investigation date range (including six from self-tests). The median age among test-positive attendees was 28 years (IQR = 23.0–35.0 years), 13 (27.7%) of 47 with reported gender were male, 15 (32.6%) of 46 with reported race/ethnicity were non-Hispanic White, and 19 (42.2%) of 45 reporting residency were New York residents. Among 47 test-positive survey respondents reporting vaccination information, 37 (78.7%) completed a primary vaccination series, six (12.8%) received a booster dose, and four (8.5%) were partially vaccinated. Among 4,157 test-negative respondents, 2,274 (54.7%) completed primary vaccination, 1,511 (36.3%) received a booster dose, and 372 (8.9%) were partially vaccinated. The median interval from booster dose receipt to a SARS-CoV-2–positive specimen was 12 days (IQR = 10–21 days) and to a negative specimen was 20 days (IQR = 10–35 days).
Among the 48 test-positive respondents, 34 (70.8%) reported COVID-19 compatible symptoms, compared with 312 (7.4%) of 4,203 test-negative respondents. Nasal congestion or runny nose (91.2%) and fatigue (88.2%) were common symptoms reported among test-positive respondents; among test-negative respondents, nasal congestion or runny nose (223 of 310; 71.9%) and sore throat (191 of 305; 62.6%) were most commonly reported. No hospitalizations were reported.
Test-positive survey respondents reported engaging in certain activities more frequently than did test-negative respondents, including attending bars (16.7% versus 6.9%), karaoke (18.8% versus 2.4%), or nightclubs (10.4% versus 3.0%) outside of the convention, and eating or drinking indoors near others for at least 15 minutes at the convention (62.5% versus 43.7%) (all p<0.05). Differences were also found in reporting close contact with someone with a positive COVID-19 test result within 10 days of symptom onset or test result (44.1% versus 6.0%) (p<0.05). Most (4,041 [95.2%]) attendees, reported always wearing a mask over their nose and mouth while indoors; no difference was found in type of mask used by test result. Among 4,245 survey respondents, 87 (2.0%) reported knowing at least one person (mean = 2.4) whom they met, interacted with, or worked with during the event who received a positive SARS-CoV-2 test result since attending the event.
On December 2, 2021, after identification of the first Omicron case, CDC issued an Epidemic Information Exchange (Epi-X) notification to U.S. health departments to identify COVID-19 cases among event attendees. On December 3, 2021, the NYC Test and Trace program§§§ alerted registered attendees via text and email messages to get tested immediately, wear a face mask, and maintain physical distance from others.
Discussion
This investigation identified 119 event-associated COVID-19 cases, including one hospitalization. A parallel epidemiologic investigation describing a cluster of attendees with social links (2) revealed that at least seven U.S.-based persons potentially attended the event during their infectious period.¶¶¶ Despite these potential exposures and multiple introductions as evidenced by genomic identification of at least three different SARS-CoV-2 variants and sublineages, findings from surveillance and survey data from a portion of attendees suggest that this large event did not lead to widespread transmission; 7-day average percentage of positive test results in NYC on December 5, 2021, (3.0%) was similar to that in this investigation (2.6%) (3). Omicron variant accounted for <5% of sequenced cases in NYC by December 4, 2021; transmission could have been higher had the convention occurred after Omicron became the dominant variant (4).
Reported prevention measures (vaccination requirements, enforcement of mask use, and avoidance of unmasked indoor settings), and a venue with HEPA filtration likely accounted for the limited number of event-associated cases. Indoor gatherings in which prevention measures do not occur have been shown to increase the spread of COVID-19 (5–8). In addition, transmission to household contacts, including to vaccinated or previously infected persons, was documented in the related cluster investigation and a previous Omicron investigation (2,9).
The findings in this report are subject to at least six limitations. First, case finding and survey distribution were limited to a registration list of 35,613 ticket purchasers, but the event organizer reported that approximately 53,000 persons had attended. Second, matching attendees with case surveillance data was conducted by jurisdictions using only name and address, which potentially limited the number of cases and vaccination records identified or misidentified attendees. In addition, self-testing results were not included by most jurisdictions. Third, few specimens were available for sequencing (17% of event-associated cases). Fourth, the limited reach (14% of reported attendees) and low response rate of the survey (approximately 21%) can increase potential biases if respondents differ systematically from nonrespondents. Fifth, responses were subject to self-reporting bias; attendees who sought testing might be more likely to respond or respond according to social desirability bias. Finally, the definition of event-association case could have included cases from transmission unrelated to the event.
Findings from this survey and a related cluster investigation (2) of a portion of attendees suggest transmission occurred primarily among social circles and during indoor unmasked activities during the event rather than at official event activities. These findings reinforce the importance of implementing multiple, simultaneous prevention measures, such as ensuring up-to-date vaccination, mask use, physical distancing, and improved ventilation in limiting SARS-CoV-2 transmission, including highly transmissible Delta and Omicron variants, during large indoor events.
Acknowledgments
Ben Brumfield, Sheryl Roehl, Mona Byrkit, Nicole Fehrenbach, Anna Llewellyn, Rieza Soelaeman, Alicia Dunajcik, Charles Braxton, Chisom Onyeuku, Christina Winfield, Cody Bennett, Denise Sheriff, Francisco Palomeque, Geremy Lloyd, Isaa Lee-Hall, Laird Ruth, Laura Hill, Lauren Billick, Namita Agravat, Neela Persad, Otto Ike, Rebecca Sabo, Robert Amy, Tomi Ademokun, Veneranda Ngulefac, CDC COVID-19 Emergency Response Team; Clarion Events; Shama Ahuja, Jennifer Baumgartner, Elizabeth Luoma, Emily McGibbon, Don Weiss, New York City Department of Health and Mental Hygiene; New York City Test and Trace Corps; Stella Tsai, Troy Brancard, Lindsay Lowe, New Jersey Department of Health; Lynn Sosa, Connecticut Department of Public Health; Jyoti Narayana, Marilee Kellis Butterfield, Arizona Department of Health Services; Christian Santiago, Puerto Rico Department of Health; Kirsten St. George, Jennifer Laplante, Patrick Bryant, Amy Dean, Meghan Fuschino, Alexis Russell, New York State Department of Health.
Corresponding author: Libby Horter, qsw2@cdc.gov.
1CDC COVID-19 Emergency Response Team; 2Goldbelt C6, LLC, Chesapeake, Virginia; 3Oak Ridge Institute for Science and Education, Oak Ridge, Tennessee; 4New York City Department of Health and Mental Hygiene, New York; 5New York State Department of Health; 6Minnesota Department of Health; 7Connecticut Department of Public Health; 8Epidemic Intelligence Service, CDC; 9Massachusetts Department of Public Health; 10Pennsylvania Department of Health; 11CSTE Applied Fellow, Council State and Territorial Epidemiologists, Atlanta, Georgia; 12Philadelphia Department of Public Health, Philadelphia, Pennsylvania; 13Division of Disease Control and Health Protection, Florida Department of Health; 14New Jersey Department of Health; 15Rhode Island Department of Health; 16North Carolina Department of Health and Human Services; 17Delaware Division of Public Health, Dover, Delaware; 18Virginia Department of Health; 19California Department of Public Health; 20South Carolina Department of Health & Environmental Control; 21Michigan Department of Health and Human Services; 22Tennessee Department of Health; 23Division of State and Local Readiness, Office of Public Health Preparedness and Response, CDC; 24Wisconsin Department of Health Services; 25Arizona Department of Health Services; 26Chicago Department of Public Health, Chicago, Illinois; 27Nevada Department of Health and Human Services; 28Hawaii State Department of Health; 29Missouri Department of Health and Senior Services; 30Arkansas Department of Health; 31Colorado Department of Public Health and Environment; 32Idaho Department of Health and Welfare; 33Ohio Department of Health;34Indiana Department of Health; 35CDC Foundation, Atlanta, Georgia; 36Kentucky Department of Health; 37Maine Department of Health and Human Services; 38New Hampshire Division of Public Health Services; 39Utah Department of Health; 40West Virginia Department of Health & Human Resources; 41Mississippi State Department Health; 42State of Montana Department of Health and Human Services;43Nebraska Department of Health and Human Services; 44Puerto Rico Department of Health; 45North Dakota Department of Health.
All authors have completed and submitted the International Committee of Medical Journal Editors form for disclosure of potential conflicts of interest. Ruth Lynfield reports unpaid positions as the President of the Council of State and Territorial Epidemiologists and on the National Foundation for Infectious Diseases Executive Board. Ruby Serrano reports honoraria from Ponce Health Sciences University. No other potential conflicts of interest were disclosed.
† https://www.cdc.gov/coronavirus/2019-ncov/daily-life-coping/index.html
§ Persons who had received a primary vaccination series had completed all recommended doses of a Food and Drug Administration–authorized COVID-19 vaccine (2 doses of BNT162b2 [Pfizer-BioNTech] or mRNA-1273 [Moderna], or 1 dose of Ad.26.COV2.S (Janssen [Johnson & Johnson]) ≥14 days before specimen collection and had documentation in their state immunization information system or self-report of vaccination details (including vaccine product and dates of receipt). Persons who had received a booster dose had completed a primary vaccination series and received another dose of vaccination regardless of the time frame. Persons who had received only 1 dose of a 2-dose vaccination series or had completed vaccination <14 days before specimen collection were considered partially vaccinated.
¶ Sequencing platforms included Nanopore (Oxford Nanopore Technologies); NextSeq (Illumina); NovaSeq 6000 (Illumina); Miseq System (Illumina); PacBio Sequel II Systems (PacBio); and GridION (Oxford Nanopore Technologies).
** COVID-19–like symptoms were based on the Council of State and Territorial Epidemiologists surveillance case definition for COVID-19. https://ndc.services.cdc.gov/case-definitions/coronavirus-disease-2019-2020-08-05/
†† Respondents who did not confirm attending the convention or who resided outside the United States were excluded from the analysis.
§§ 45 C.F.R. part 46.102(l)(2); 21 C.F.R. part 56; 42 U.S.C. Sect. 241(d); 5 U.S.C. Sect. 552a; 44 U.S.C. Sect. 3501 et seq.
¶¶ https://www.cdc.gov/coronavirus/2019-ncov/testing/self-testing.html
*** https://www.cdc.gov/coronavirus/2019-ncov/vaccines/booster-shot.html
††† The closely linked Omicron cases involved lineage BA.1 SARS-CoV-2 with no discernable difference in the genomic regions with reliable sequence, consistent with the epidemiologic links.
§§§ https://www.nychealthandhospitals.org/test-and-trace/external icon
¶¶¶ Infectious period for the cluster investigations was defined as 2 days before and 10 days after their symptom onset date.
References
- Minnesota Department of Health. Lab testing confirms state’s first COVID-19 case involving Omicron variant [Press release]. Saint Paul, MN: Minnesota Department of Health; 2021. https://www.health.state.mn.us/news/pressrel/2021/covid120221.htmlexternal icon
- Smith-Jeffcoat SE, Pomeroy MA, Sleweon S, et al. Multistate outbreak of SARS-CoV-2 B.1.1.529 (Omicron) variant infections among persons in a social network attending a convention—New York City, November 18–December 20, 2021. MMWR Morb Mortal Wkly Rep 2022;71.
- NYC Health. COVID-19: data. New York, NY: NYC Health; 2022. Accessed January 13, 2022. https://www1.nyc.gov/site/doh/covid/covid-19-data.page#testingexternal icon
- NYC Health. Coronavirus data: variants. New York, NY: NYC Health; 2022. Accessed January 13, 2022. https://github.com/nychealth/coronavirus-data/blob/master/variants/now-variant-epi-data.csvexcel iconexternal icon
- Brown CM, Vostok J, Johnson H, et al. Outbreak of SARS-CoV-2 infections, including COVID-19 vaccine breakthrough infections, associated with large public gatherings—Barnstable County, Massachusetts, July 2021. MMWR Morb Mortal Wkly Rep 2021;70:1059–62. https://doi.org/10.15585/mmwr.mm7031e2external icon PMID:34351882external icon
- Hamner L, Dubbel P, Capron I, et al. High SARS-CoV-2 attack rate following exposure at a choir practice—Skagit County, Washington, March 2020. MMWR Morb Mortal Wkly Rep 2020;69:606–10. https://doi.org/10.15585/mmwr.mm6919e6external icon PMID:32407303external icon
- Sami S, Turbyfill CR, Daniel-Wayman S, et al. Community transmission of SARS-CoV-2 associated with a local bar opening event—Illinois, February 2021. MMWR Morb Mortal Wkly Rep 2021;70:528–32. https://doi.org/10.15585/mmwr.mm7014e3external icon PMID:33830981external icon
- Muller N, Kunze M, Steitz F, et al. Severe acute respiratory syndrome coronavirus 2 outbreak related to a nightclub, Germany, 2020. Emerg Infect Dis 2020;27:645–8. https://doi.org/10.3201/eid2702.204443external icon PMID:33263514external icon
- Jansen L, Tegomoh B, Lange K, et al. Investigation of a SARS-CoV-2 B.1.1.529 (Omicron) variant cluster—Nebraska, November–December 2021. MMWR Morb Mortal Wkly Rep 2021;70:1782–4. https://doi.org/10.15585/mmwr.mm705152e3external icon PMID:34968376external icon
 FIGURE. Event-associated cases* of SARS-CoV-2 infection (n = 119)† among attendees of a large indoor convention in New York City, by date of specimen collection and test type§ — 16 jurisdictions, November–December 2021
FIGURE. Event-associated cases* of SARS-CoV-2 infection (n = 119)† among attendees of a large indoor convention in New York City, by date of specimen collection and test type§ — 16 jurisdictions, November–December 2021
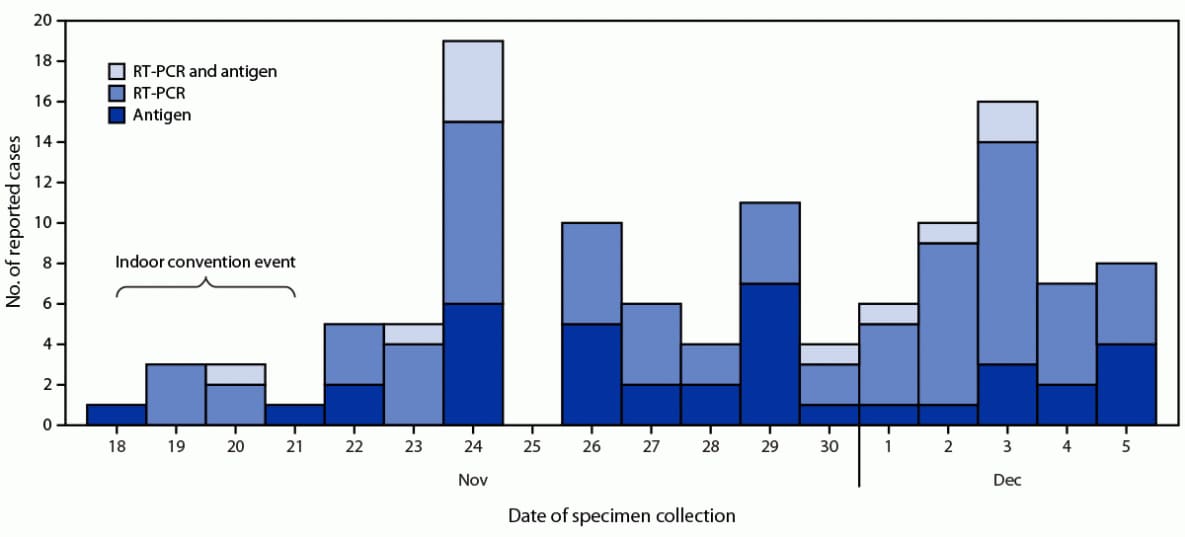
Abbreviation: RT-PCR = reverse transcription–polymerase chain reaction.
* Reported by health department COVID-19 surveillance systems.
† Among 4,560 attendees with test result data, 119 (2.6%) event-associated cases were identified by January 6, 2022, from 16 jurisdictions.
§ Antigen, RT-PCR, and RT-PCR and antigen are mutually exclusive groups.
Abbreviation: NA = not applicable.
* Testing for statistical significance was conducted using nonparametric tests (i.e., Wilcoxon rank-sum) to compare continuous data and Pearson’s chi-square or Fisher’s exact test to compare categorical data. Statistical significance was defined as p<0.05.
† “Known no.” is defined as number of persons for individual variables that did not have missing data.
§ Percentage among those who received a booster dose.
¶ Percentage among those who received a primary vaccination series.
Abbreviations: NA = not applicable; NYC = New York City.
* Self-reported SARS-CoV-2 test results from an online, anonymous survey.
† Testing for statistical significance was conducted using nonparametric tests (i.e., Wilcoxon rank-sum) to compare continuous data and Pearson’s chi-square or Fisher’s exact test to compare categorical data. Statistical significance was defined as p<0.05.
§ Known no. is defined as number of persons for individual variables that did not have missing data.
¶ Other is defined in the survey as “none of these.”
** COVID Alert NYC app is a voluntary, anonymous, exposure-notification smartphone application that provides an alert if a person was in close contact with someone who receives a positive SARS-CoV-2 test result. The period for exposure notification was November 18–December 5, 2021.
Suggested citation for this article: Sami S, Horter L, Valencia D, et al. Investigation of SARS-CoV-2 Transmission Associated With a Large Indoor Convention — New York City, November–December 2021. MMWR Morb Mortal Wkly Rep 2022;71:243–248. DOI: http://dx.doi.org/10.15585/mmwr.mm7107a4external icon.
MMWR and Morbidity and Mortality Weekly Report are service marks of the U.S. Department of Health and Human Services.
Use of trade names and commercial sources is for identification only and does not imply endorsement by the U.S. Department of
Health and Human Services.
References to non-CDC sites on the Internet are
provided as a service to MMWR readers and do not constitute or imply
endorsement of these organizations or their programs by CDC or the U.S.
Department of Health and Human Services. CDC is not responsible for the content
of pages found at these sites. URL addresses listed in MMWR were current as of
the date of publication.
All HTML versions of MMWR articles are generated from final proofs through an automated process. This conversion might result in character translation or format errors in the HTML version. Users are referred to the electronic PDF version (https://www.cdc.gov/mmwr) and/or the original MMWR paper copy for printable versions of official text, figures, and tables.
Questions or messages regarding errors in formatting should be addressed to mmwrq@cdc.gov.