Description of 13 Infants Born During October 2015–January 2016 With Congenital Zika Virus Infection Without Microcephaly at Birth — Brazil
Weekly / December 2, 2016 / 65(47);1343–1348
On November 22, 2016, this report was posted online as an MMWR Early Release.
Vanessa van der Linden, MD1*; André Pessoa, MD2*; William Dobyns, MD3; A. James Barkovich, MD4; Hélio van der Linden Júnior, MD5; Epitacio Leite Rolim Filho, MD, PhD1,6; Erlane Marques Ribeiro, MD, PhD2; Mariana de Carvalho Leal, MD, PhD6,7; Pablo Picasso de Araújo Coimbra, MD8; Maria de Fátima Viana Vasco Aragão, MD, PhD9,10; Islane Verçosa, MD11; Camila Ventura, MD, PhD12,13; Regina Coeli Ramos, MD12; Danielle Di Cavalcanti Sousa Cruz, MD13; Marli Tenório Cordeiro, PhD14; Vivian Maria Ribeiro Mota15; Mary Dott, MD16; Christina Hillard, MA17; Cynthia A. Moore, MD, PhD17 (View author affiliations)
View suggested citationSummary
What is already known about this topic?
Congenital Zika virus infection can cause microcephaly and severe brain abnormalities. As more information about the associated clinical syndrome becomes available, the phenotype is expanding to include other, sometimes less severe features, such as brain abnormalities without congenital microcephaly.
What is added by this report?
Although infants with congenital Zika virus infection who have a normal head size have been described in large series, sufficient description of the features of congenital Zika syndrome in these infants has not been available. This report of a series of 13 infants with laboratory evidence of congenital Zika virus infection with normal head size at birth includes the findings from extensive imaging, neurologic, ophthalmologic, auditory, and orthopedic examinations. Follow-up of these infants has shown that for most, head growth deceleration occurs to the point of microcephaly after birth and significant neurologic sequelae are evident.
What are the implications for public health practice?
Additional information is needed to fully describe the spectrum of findings associated with congenital Zika virus infection; however, microcephaly might not be evident at birth but can develop after birth in infants with underlying brain abnormalities. These findings underscore the importance of early neuroimaging for infants exposed to Zika virus prenatally.
Altmetric:
Congenital Zika virus infection can cause microcephaly and severe brain abnormalities (1). Congenital Zika syndrome comprises a spectrum of clinical features (2); however, as is the case with most newly recognized teratogens, the earliest documented clinical presentation is expected to be the most severe. Initial descriptions of the effects of in utero Zika virus infection centered prominently on the finding of congenital microcephaly (3). To assess the possibility of clinical presentations that do not include congenital microcephaly, a retrospective assessment of 13 infants from the Brazilian states of Pernambuco and Ceará with normal head size at birth and laboratory evidence of congenital Zika virus infection was conducted. All infants had brain abnormalities on neuroimaging consistent with congenital Zika syndrome, including decreased brain volume, ventriculomegaly, subcortical calcifications, and cortical malformations. The earliest evaluation occurred on the second day of life. Among all infants, head growth was documented to have decelerated as early as 5 months of age, and 11 infants had microcephaly. These findings provide evidence that among infants with prenatal exposure to Zika virus, the absence of microcephaly at birth does not exclude congenital Zika virus infection or the presence of Zika-related brain and other abnormalities. These findings support the recommendation for comprehensive medical and developmental follow-up of infants exposed to Zika virus prenatally. Early neuroimaging might identify brain abnormalities related to congenital Zika infection even among infants with a normal head circumference (4).
Thirteen infants with laboratory evidence of congenital Zika virus infection and normal head size (less than or equal to 2 standard deviations [SD] below the mean for sex and gestational age) at birth (during October 2015–January 2016) are included in this report. The infants were evaluated by multidisciplinary teams at two referral centers in Brazil: the Rehabilitation Center of Association for Assistance of Disabled Children of Pernambuco, Recife, Pernambuco State, and the Infantil Albert Sabin Hospital, Fortaleza, Ceará State during the months of October 2015–October 2016.
Eleven of the infants came to clinical attention because their birth head circumference was below the level established by the Brazilian Ministry of Health as requiring further evaluation for possible congenital Zika virus infection (http://combateaedes.saude.gov.br/images/sala-de-situacao/Microcefalia-Protocolo-de-vigilancia-e-resposta-10mar2016-18h.pdfpdf iconexternal icon). This level was 33 cm before December 2, 2015, and 32 cm for gestational age ≥37 weeks after that date; however, all of these infants had head circumferences at birth that did not exceed 2 SD below the mean for gestational age, and therefore did not meet the definition for microcephaly (more than 2 SD below the mean). These 11 infants were referred for neurologic evaluation and neuroimaging. The remaining two infants who had head circumferences in the normal range at birth were referred for neurologic evaluation at ages 5 and 7 months because of developmental concerns.
A standard form was used to collect demographic and clinical information, including whether the mothers recalled having had a rash during pregnancy. All information was obtained as part of the clinical protocol or as the result of clinical indication. Informed consent was obtained for the collection, use, and publication of clinical photographs of the infants.
Laboratory evidence of congenital Zika virus infection was defined as negative laboratory test results for five infectious causes of congenital microcephaly (toxoplasmosis, cytomegalovirus, rubella, syphilis and human immunodeficiency virus) and serologic evidence of Zika virus infection (a positive Zika virus-specific immunoglobulin M (IgM) capture enzyme–linked immunosorbent assay (MAC-ELISA) result on infant cerebrospinal fluid [CSF] or serum). Conventional reverse transcription–polymerase chain reaction (RT-PCR) was performed for the detection of Zika virus and dengue virus RNA, and real-time RT-PCR was performed for chikungunya virus in the Recife location. Monoplex real-time RT-PCR for Zika virus was performed in the Fortaleza location (5,6). Maternal testing for evidence of Zika virus infection was not available during the time of the 13 pregnancies.
For this report, microcephaly was defined as head circumference (HC) (also known as occipitofrontal circumference) more than 2 SD below the mean for gestational age and sex, according to the Fetal International and Newborn Growth Consortium for the 21st Century (INTERGROWTH-21st) for fetal and newborn growth (https://intergrowth21.tghn.org/external icon) and the World Health Organization Child Growth Standards for infants (www.who.int/childgrowth/en/external icon). Birth weight was evaluated and classified as appropriate, small, or large for gestational age and sex, also using INTERGROWTH-21st standards.
All infants had clinical neurologic and orthopedic evaluations, and brain imaging with computerized tomography (CT) scan without contrast, magnetic resonance imaging (MRI) without contrast, or both, and radiographs of the hips to identify congenital dislocation. In addition, all infants had clinical noninstrumental evaluation of dysphagia by a speech therapist, ophthalmologic examination with ophthalmoscopy assessment, and 11 of 13 infants had auditory evaluation by screening (auditory short latency brainstem evoked response [ABR] to click stimuli) and diagnostic tests (confirmatory frequency-specific ABR with tone burst stimuli) using the routine recommended by Brazilian Heath Ministry and the American Academy of Pediatrics Joint Committee on Infant Hearing (7). Infants clinically suspected of having seizure activity had an electroencephalogram to confirm.
The case series included nine male infants and four female infants (Table 1). Eleven patients were born at term (37–41 weeks’ gestation) and two were preterm (35 and 36 weeks’ gestation). Six of 13 mothers described a cutaneous rash between the second and fifth months of pregnancy. All infants had birth weights that were appropriate for gestational age (i.e., within 2 SD of the mean for sex and gestational age). Craniofacial disproportion was noted in six infants; three had redundant skin on the scalp at birth. Three infants had hip dysplasia, including one infant with arthrogryposis who had bilateral dislocated hips.
All infants had positive tests for Zika virus–specific IgM in either CSF only (nine infants), serum only (two infants) or CSF and serum (two infants). Seven CSF specimens were tested for Zika virus RNA by RT-PCR and all were negative; two of these infants also had negative RT-PCR testing in serum collected at the same time as CSF. RT-PCR testing results on the two serum-only specimens are pending. No Zika virus testing was performed on urine. Most infants (eight of 13) were tested within the first month of life; however, the date of testing of CSF for two infants is not known. Three infants were tested for Zika virus outside the neonatal period. Although identified at birth because of head size, one infant was not tested until age 4 months; two infants were tested at ages 5 months and 7 months when they were first evaluated because of developmental delay. One infant with both CSF and serum IgM testing positive at birth tested negative on serum re-testing at 6 months of age; another remained positive on re-testing at age 5 months.
HCs at birth ranged from 0.30 to -2.00 SD from the mean for gestational age and sex (Table 1). All infants showed a decrease in the rate of HC growth between birth and the time of the last examination. In 11 of 13 infants, postnatal microcephaly was diagnosed because of an HC measurement more than 2 SD below the mean for age and sex. Neuroimaging (CT scan in 13 infants and MRI in 10 infants) showed malformations of cortical development, which were most predominant anteriorly, and calcifications, predominantly in the subcortical region (especially in the transition area between the cortex and white matter). All neuroimaging showed evidence of decreased brain volume, with ventriculomegaly in all infants, and increased extra-axial CSF space in two of 13 infants (Table 2) (Figure).
Dysphagia was found on clinical evaluation in 10 of 13 infants. Seven infants had a diagnosis of epilepsy. Five infants had some degree of irritability, which improved by age 4 months. Most infants (12 of 13) had good visual interaction; one infant exhibited no eye contact. Three of 13 infants had chorioretinal abnormalities. All 11 infants tested had normal hearing evaluations. All infants had some degree of hypertonia; 12 of 13 had pyramidal and extrapyramidal signs with dystonic movement. One infant had spastic hemiparesis and another had bilateral hemiparesis, more severe on the left side. One infant with arthrogryposis was difficult to assess because of increased tone in some muscles and decreased in others. Nine of 13 infants had no voluntary movement of the hands and had a grasp reflex. Good head control was present in eight of 13 infants (supplemental material at https://stacks.cdc.gov/view/cdc/42517).
Discussion
Congenital microcephaly has been a hallmark of intrauterine infection with Zika virus. However, despite the absence of microcephaly at birth, the 13 infants in this report with laboratory evidence of Zika virus infection had brain abnormalities associated with congenital Zika syndrome, including ventriculomegaly and decreased brain volume, cortical malformations and subcortical calcifications, underscoring the importance of neuroimaging in evaluating these infants. In addition some infants had other structural or functional abnormalities that might have brought them to medical attention regardless of their head size; however, these findings did not occur more frequently in infants with the smallest HCs. Congenital Zika virus infection without microcephaly at birth previously has been reported (8), as has postnatal development of microcephaly in infants presumed to be infected congenitally (9). However, this is the first series of infants with laboratory evidence of congenital Zika virus infection documented to have poor head growth with microcephaly developing after birth.
Decreases in the rate of head growth postnatally in these infants was accompanied by significant neurologic dysfunction, including hypertonia and hemiparesis, dyskinesia/dystonia, dysphagia, epilepsy, and persistence of primitive reflexes. Although these neurologic findings are consistent with previous reports of infants with congenital microcephaly who had prenatal exposure to Zika virus (2), infants who did not have microcephaly at birth showed better social interaction (i.e., they made and held eye contact and had a social smile). However, more than 60% of infants in this series had epilepsy (likely related to the cortical malformations), and all had significant motor disabilities consistent with mixed cerebral palsy (10). The infants were too young to be adequately assessed for cognitive deficits.
Among the six mothers who reported a rash, four reported rash in the first trimester and two in the second trimester. Therefore, among these mothers, early occurrence of the presumed infection during pregnancy did not result in the most severe congenital Zika phenotype (i.e., microcephaly at birth). Only three infants were reported to have a history of prenatal ultrasound abnormalities consistent with congenital Zika virus infection.
The pathogenesis of postnatal microcephaly from congenital Zika virus infections is not known. The decrease in head growth might be the consequence of earlier in utero destruction of neuroprogenitor or other neural cells, persistent inflammatory response-associated molecules, or continued infection of neural cells. The last seems less likely given the negative Zika virus RT-PCR results in all seven tested CSF samples.
The findings in this report are subject to at least four limitations. First, birth HC measurements were recorded with a resolution of 0.5 cm (in contrast to the customary 0.1 cm), which likely resulted in either overestimate or underestimate of the measure in some infants. Two infants had a birth HC that was at or slightly less than 2 SD below the mean, and their HCs could have been misclassified as falling within the normal range. In addition, calculations of SD can vary among the published growth standards for HC. Second, Zika IgM testing was not confirmed by plaque reduction neutralization testing; therefore, infants could have been misclassified because of cross-reactivity with other flaviviruses or nonspecific reactivity within the ELISA. Third, based on this clinical series alone, the birth prevalence of congenital Zika syndrome without microcephaly in the population cannot be estimated. Finally, because serial neuroimaging in an infant is not clinically indicated, progressive changes such as the rate of brain volume loss cannot be assessed.
Additional information is needed to fully describe the clinical spectrum of findings associated with congenital Zika virus infection. This report documents that microcephaly at birth is not an essential hallmark of congenital Zika syndrome. Infants with normal HC at birth have brain and other abnormalities associated with congenital Zika syndrome and might develop microcephaly after birth. These findings demonstrate the importance of early neuroimaging for infants exposed to Zika virus prenatally and the need for comprehensive medical and developmental follow-up.
Corresponding author: Cynthia A. Moore, zikamch@cdc.gov; 770-488-7100.
1Association for Assistance of Disabled Children, Recife, Pernambuco, Brazil; 2Hospital Infantil Albert Sabin, Fortaleza, Ceará, Brazil; 3University of Washington and Seattle Children’s Research Institute, Seattle; 4University of California-San Francisco; 5Dr. Henrique Santillo Rehabilitation Center, Goiania, Brazil; 6Federal University of Pernambuco, Recife, Pernambuco, Brazil; 7Agamenon Magalhães Hospital (HAM), Recife, Pernambuco Brazil; 8Uniclinic Diagnóstico por Imagem, Fortaleza, Brazil; 9Centro Diagnóstico Multimagem, Recife, Pernambuco, Brazil; 10Mauricio de Nassau University, Recife, Pernambuco, Brazil; 11Caviver Clinical, Fortaleza, Ceará, Brazil; 12Altino Ventura Foundation, Recife, Pernambuco, Brazil; 13Pernambuco’s Eye Hospital, Recife, Pernambuco, Brazil; 12Oswaldo Cruz University Hospital, Recife, Pernambuco, Brazil; 13Professor Fernando Figueira Integral Medicine Institute, Recife, Pernambuco, Brazil; 14Centro de Pesquisas Aggeu Magalhães-Fiocruz, Recife, Pernambuco, Brazil; 15University of Fortaleza, Fortaleza, Brazil; 16Center for Surveillance, Epidemiology and Laboratory Services, CDC; 17National Center on Birth Defects and Developmental Disabilities, CDC.
* These authors contributed equally to this report.
References
- de Araújo TV, Rodrigues LC, de Alencar Ximenes RA, et al. Association between Zika virus infection and microcephaly in Brazil, January to May, 2016: preliminary report of a case-control study. Lancet Infect Dis 2016. Epub September 15, 2016. PubMedexternal icon
- Moore CA, Staples JE, Dobyns WB, et al. Characterizing the pattern of anomalies in congenital Zika syndrome for pediatric clinicians. JAMA Pediatr 2016. CrossRefexternal icon PubMedexternal icon
- Schuler-Faccini L, Ribeiro EM, Feitosa IM, et al. Possible association between Zika virus infection and microcephaly—Brazil, 2015. MMWR Morb Mortal Wkly Rep 2016;65:59–62. CrossRefexternal icon PubMedexternal icon
- Russell K, Oliver SE, Lewis L, et al. Update: interim guidance for the evaluation and management of infants with possible congenital Zika virus infection—United States, August 2016. MMWR Morb Mortal Wkly Rep 2016;65:870–8. CrossRefexternal icon PubMedexternal icon
- Lanciotti RS, Calisher CH, Gubler DJ, Chang GJ, Vorndam AV. Rapid detection and typing of dengue viruses from clinical samples by using reverse transcriptase-polymerase chain reaction. J Clin Microbiol 1992;30:545–51. PubMedexternal icon
- Lanciotti RS, Kosoy OL, Laven JJ, et al. Genetic and serologic properties of Zika virus associated with an epidemic, Yap State, Micronesia, 2007. Emerg Infect Dis 2008;14:1232–9. CrossRefexternal icon PubMedexternal icon
- American Academy of Pediatrics, Joint Committee on Infant Hearing. Year 2007 position statement: principles and guidelines for early hearing detection and intervention programs. Pediatrics 2007;120:898–921. CrossRefexternal icon PubMedexternal icon
- França GV, Schuler-Faccini L, Oliveira WK, et al. Congenital Zika virus syndrome in Brazil: a case series of the first 1501 livebirths with complete investigation. Lancet 2016;388:891–7. CrossRefexternal icon PubMedexternal icon
- Moura da Silva AA, Ganz JSS, Sousa PD, et al. Early growth and neurologic outcomes of infants with probable congenital Zika virus syndrome. Emerg Infect Dis 2016;22:1953–6. CrossRefexternal icon PubMedexternal icon
- Wimalasundera N, Stevenson VL. Cerebral palsy. Pract Neurol 2016;16:184–94. CrossRefexternal icon PubMedexternal icon
 TABLE 1. Clinical history and physical findings from 13 infants with congenital Zika infection without microcephaly at birth — Brazil, October 2015–October 2016
TABLE 1. Clinical history and physical findings from 13 infants with congenital Zika infection without microcephaly at birth — Brazil, October 2015–October 2016
| Patient no. | Sex | Gestational age (wks) | Birth weight (gm) | Reported prenatal ultrasound abnormalities* | Maternal rash | Infant Zika virus IgM antibody by ELISA | Birth HC (cm) and (SD†) | Age (mos) at last follow-up | Follow-up HC (cm) and (SD§) | Ocular findings¶ | Craniofacial disproportion at birth** | Arthrogryposis or hip dysplasia at birth†† |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | F | 35 | 2,570 | no | 3 mo. | CSF, serum + | 29.5 (-1.72) | 11 | 39 (-3.86) | no | yes | no |
| 2 | M | 38 | 3,125 | yes | 2 mo. | CSF + | 33.0 (-0.40) | 10 | 41 (-3.33) | yes | no | yes |
| 3 | M | 39 | 2,770 | no | none | CSF, serum + | 32.0 (-1.63) | 11 | 43 (-2.11) | no | no | no |
| 4 | M | 37 | 2,785 | yes | 2 mo. | CSF + | 31.0 (-1.65) | 10 | 43 (-1.98) | no | yes | no |
| 5 | F | 37 | 2,465 | yes | 5 mo. | CSF + | 31.0 (-1.39) | 12 | 36 (-6.18) | no | yes | no |
| 6 | M | 39 | 2,975 | no | 4 mo. | CSF + | 33.0 (-0.78) | 11 | 42 (-2.89) | no | no | no |
| 7 | M | 39 | 3,840 | no | none | CSF + | 33.0 (-0.78) | 12 | 40 (-4.68) | no | yes | yes |
| 8 | F | 41 | 2,955 | no | none | CSF + | 32.0 (-1.95) | 9 | 39.5 (-3.17) | no | no | yes |
| 9 | M | 39 | 3,155 | no | 3 mo. | CSF + | 33.5 (-0.35) | 11 | 42.5 (-2.50) | no | no | no |
| 10 | M | 40 | 3,100 | no | none | CSF + | 32.0 (-2.00) | 10 | 40 (-4.27) | yes | yes | no data |
| 11 | M | 38 | 2,965 | no | none | CSF + | 33.5 (0.02) | 7 | 40 (-2.98) | no | no | no data |
| 12 | F | 36 | 2,930 | no | none | serum + | 32.5 (0.30) | 7 | 40.5 (-1.35) | no | no | no |
| 13 | M | 40 | 2,990 | no | none | serum + | 33.0 (-1.16) | 5 | 40 (-2.12) | yes | yes | no |
Abbreviations: CSF = cerebrospinal fluid; ELISA = enzyme-linked immunosorbent assay; F = female; HC = head circumference; IgM = immunoglobulin M; M = male; SD = standard deviation.
* Abnormalities include brain calcifications (patient 2), microcephaly (patient 4), and decreased brain volume with ventriculomegaly (patient 5).
† Standard deviations calculated using INTERGROWTH-21st Newborn Size Application Tool (https://intergrowth21.tghn.org/global-perinatal-package/intergrowth-21st-comparison-application/external icon).
§ Standard deviations calculated using PediTools for World Health Organization growth standard for age 0–24 months (http://peditools.org/growthwho/index.phpexternal icon).
¶ Abnormalities include macular chorioretinal atrophic lesion in right eye (patient 2), discrete chorioretinal macular atrophy in left eye (patient 10), and macular atrophy in left eye (patient 13).
** Abnormalities include redundant scalp (patients 5, 10, 13).
†† Abnormalities include arthrogryposis (patient 2), hip dysplasia (patients 2, 7, 8) and diaphragmatic weakness (patient 2).
 TABLE 2. Neuroimaging findings by computerized tomography (CT) and magnetic resonance (MR) for 13 infants with congenital Zika infection without microcephaly at birth — Brazil, October 2015–October 2016
TABLE 2. Neuroimaging findings by computerized tomography (CT) and magnetic resonance (MR) for 13 infants with congenital Zika infection without microcephaly at birth — Brazil, October 2015–October 2016
| Patient no. | Type of imaging* (age performed) | Decreased brain volume | Malformations of cortical development | Most affected lobes† | Cerebellum or brainstem hypoplasia | Corpus callosum hypoplasia† | Ventriculo-megaly | Increased extra-axial CSF space | Calcifications§ |
|---|---|---|---|---|---|---|---|---|---|
| 1 | CT (1 mo.) MR (4 mo.) | yes | yes | right anterior | yes | yes | yes | no | subcortical and basal ganglia |
| 2 | CT (1 wk.) MR (2 mo.) | yes | yes | bilateral diffuse | yes | no | yes | no | subcortical, basal ganglia |
| 3 | CT (2 days) MR (1 wk.) | yes† | yes† | right diffuse | no | yes | yes | no | subcortical |
| 4 | CT (1 day) MR (7.5 mo.) | yes | yes | unknown | yes | unknown | yes | no | subcortical and basal ganglia |
| 5 | CT (3 days) MR (12 mo.) | yes | yes | bilateral diffuse | yes | yes | yes | yes | subcortical and basal ganglia |
| 6 | CT (3 days) MR (3.5 mo.) | no | yes† | bilateral anterior | no | yes | yes | no | subcortical |
| 7 | CT (2 wks.) MR (9 mo.) |
yes | yes | bilateral diffuse | no | yes | yes | yes | subcortical |
| 8 | CT (2 mo.) | yes | yes | unknown | no | yes | yes | no | subcortical and basal ganglia |
| 9 | CT (3 days) MR (6 mo.) |
yes | yes | bilateral anterior | no | no | no | no | subcortical |
| 10 | CT (3 mo.) | yes | yes | bilateral diffuse | no | not assessed | yes | no | basal ganglia |
| 11 | CT (7 mo.) | yes | yes | bilateral diffuse | no | not assessed | yes | yes | subcortical |
| 12 | CT (1 mo.) MR (9 mo.) | yes | yes | bilateral diffuse | no | yes | no | no | subcortical |
| 13 | CT (2 mo.) MR (11 mo.) | yes | yes | bilateral diffuse | no | yes | yes | no | subcortical |
Abbreviations: CT = computed tomography; MR = magnetic resonance.
* Findings for infants evaluated both by CT and MR are consistent unless otherwise noted.
† Based on MR.
§ Based on CT.
 FIGURE. Clinical photographs and magnetic resonance (MR) and computed tomography (CT) images of two infants with congenital Zika syndrome* — Brazil, October 2015–October 2016
FIGURE. Clinical photographs and magnetic resonance (MR) and computed tomography (CT) images of two infants with congenital Zika syndrome* — Brazil, October 2015–October 2016
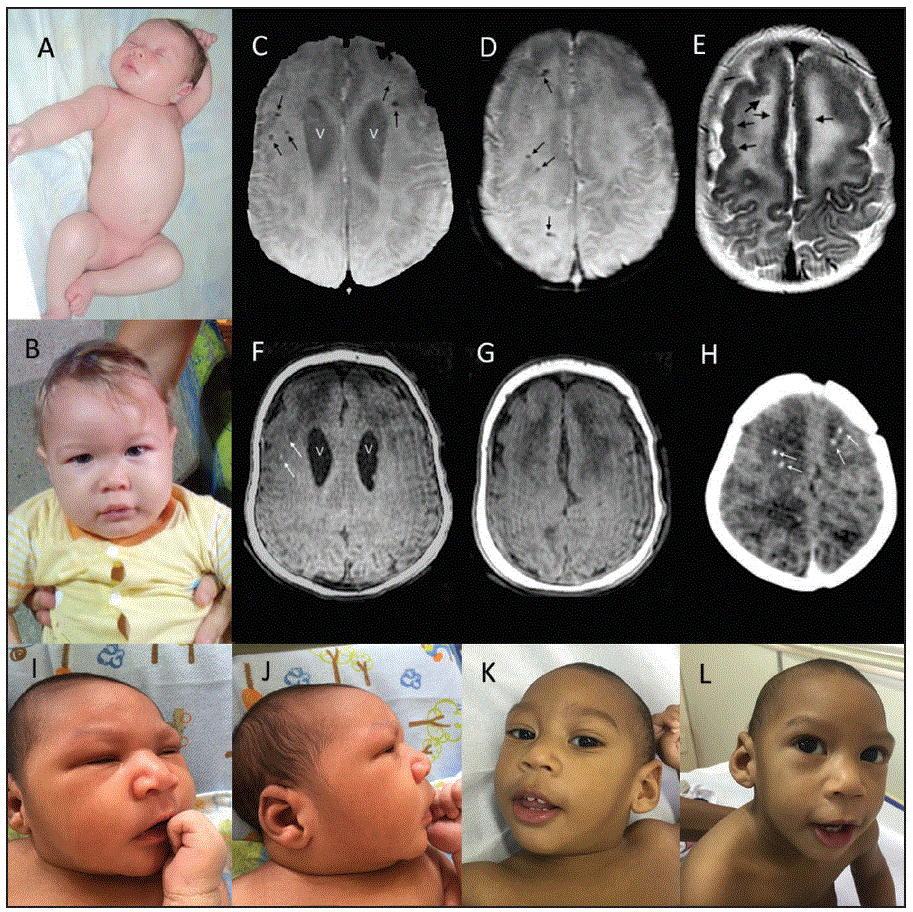
*A. A newborn (patient 6 in Table 2) with no discernable anomalies including no craniofacial disproportion and normal limbs. B. Same infant at 11 months with head circumference at almost 3 standard deviations below the mean but no apparent craniofacial anomalies. C.D. Axial susceptibility-weighted images at 3.5 months show enlarged lateral ventricles (V) and multiple calcifications (small black arrows). E. T2-weighted image shows thickened frontal cortex with reduced frontal sulcation. Slight irregularities of the inner cortical surfaces of the frontal lobes (black arrows), consistent with polymicrogyria. F.G. On T1-weighted images the ventricles (V) are slightly more apparent and two of the larger calcifications (white arrows) are seen as areas of hyperintensity. H. Noncontrast axial CT from an earlier scan at 3 days shows streak artifacts from patient motion and multiple frontal white matter calcifications (white arrows). I.J. Newborn (patient 7 in Table 2) with slightly depressed frontal regions and sloping forehead noted in I. but less evident in J., the lateral view. K.L. At 12 months, photographs show clear microcephaly but also an engaged infant with good eye contact.
Suggested citation for this article: van der Linden V, Pessoa A, Dobyns W, et al. Description of 13 Infants Born During October 2015–January 2016 With Congenital Zika Virus Infection Without Microcephaly at Birth — Brazil. MMWR Morb Mortal Wkly Rep 2016;65:1343–1348. DOI: http://dx.doi.org/10.15585/mmwr.mm6547e2external icon.
MMWR and Morbidity and Mortality Weekly Report are service marks of the U.S. Department of Health and Human Services.
Use of trade names and commercial sources is for identification only and does not imply endorsement by the U.S. Department of
Health and Human Services.
References to non-CDC sites on the Internet are
provided as a service to MMWR readers and do not constitute or imply
endorsement of these organizations or their programs by CDC or the U.S.
Department of Health and Human Services. CDC is not responsible for the content
of pages found at these sites. URL addresses listed in MMWR were current as of
the date of publication.
All HTML versions of MMWR articles are generated from final proofs through an automated process. This conversion might result in character translation or format errors in the HTML version. Users are referred to the electronic PDF version (https://www.cdc.gov/mmwr) and/or the original MMWR paper copy for printable versions of official text, figures, and tables.
Questions or messages regarding errors in formatting should be addressed to mmwrq@cdc.gov.