Persons using assistive technology might not be able to fully access information in this file. For assistance, please send e-mail to: mmwrq@cdc.gov. Type 508 Accommodation and the title of the report in the subject line of e-mail.
Progress Toward Poliomyelitis Eradication --- India, January 2007--May 2009
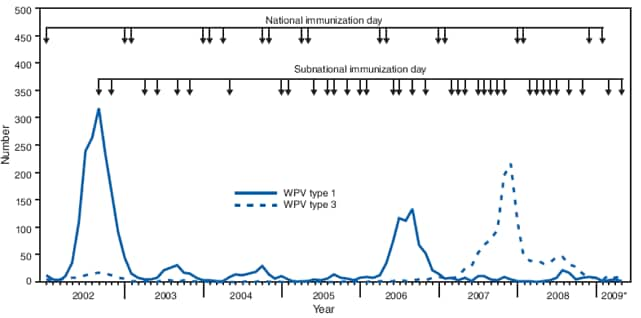
India is the most populous of the four remaining countries (including Afghanistan, Nigeria, and Pakistan) where transmission of wild poliovirus (WPV) has never been interrupted. The last cases of WPV type 2 worldwide were reported in October 1999 in India (1). However, transmission of WPV type 1 (WPV1) and WPV type 3 (WPV3) persists in India in the northern states of Uttar Pradesh and Bihar. Transmission of indigenous WPV in all of India's other states was successfully interrupted in 2002, and all WPV cases reported since then in the country have resulted from WPV circulating in Uttar Pradesh and Bihar. This report updates previous reports (1,2) and summarizes India's progress toward polio eradication since January 2007, as of May 29, 2009. In 2005, the government of India introduced the use of monovalent oral polio vaccine type 1 (mOPV1), which has higher efficacy against WPV1 than does trivalent oral polio vaccine (tOPV) (1--3), in supplementary immunization activities.* After a multistate WPV1 outbreak in 2006, preferential use of mOPV1 was accelerated and WPV1 cases decreased from 83† in 2007 to 18 during January--May 2009. A resurgence of WPV3 cases in Uttar Pradesh in 2007 led to an outbreak in Bihar. SIAs using monovalent type 3 OPV (mOPV3) were expanded in 2007 (2), and the number of WPV3 cases declined from 794 in 2007 to 41 during January--May 2009. Simultaneously interrupting transmission in high-risk areas of western Uttar Pradesh and Bihar is the key to successful interruption of all WPV transmission in India.
Immunization Activities
The routine vaccination schedule in India includes doses of tOPV at birth, 6 weeks, 10 weeks, 14 weeks, and 16--24 months. Nationally, estimated routine coverage with 3 or more doses of tOPV by age 12 months was 66% in children aged 12--23 months in 2007--2008 (4). Estimated routine coverage was 53% in Bihar and 40% in Uttar Pradesh (5).
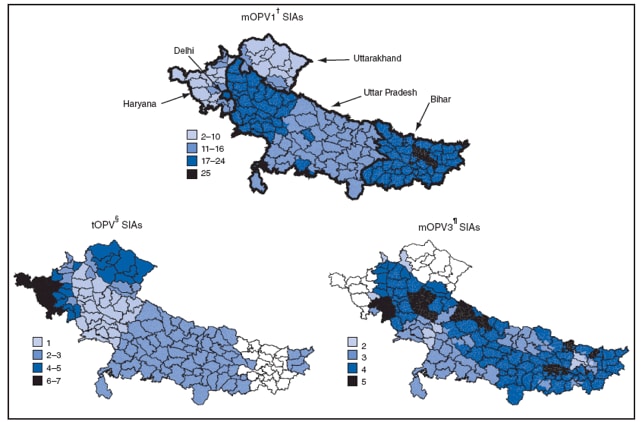
The government of India conducted two national SIA rounds each year in 2007, 2008, and 2009, which used tOPV, mOPV1, or mOPV3 in different areas depending on serotype-specific risk assessment. Additional subnational SIAs with tOPV, mOPV1, or mOPV3 were conducted in areas with ongoing transmission and mop-up activities§ with either mOPV1 or mOPV3 were conducted in areas with newly identified WPV transmission (Figure 1). Surveys conducted to assess coverage at the end of SIA activity during 2008-2009 have shown that 2%--3% of children in Uttar Pradesh and <1% of children in Bihar were missed during SIAs. SIA quality in both areas has improved from earlier periods (2). Similar surveys in the difficult to access Kosi River basin of Bihar have demonstrated that 6%--13% of children have been missed during SIAs (World Health Organization [WHO], unpublished data, 2009).
Acute Flaccid Paralysis (AFP) Surveillance
The polio eradication initiative relies on surveillance for AFP to identify poliomyelitis cases; AFP surveillance is monitored according to WHO targets for case detection and adequate stool specimen collection.¶ The national nonpolio AFP rate among children aged <15 years was 9.4 per 100,000 in 2007, 10.2 per 100,000 in 2008, and 6.6 per 100,000 during January--May 2009. In Bihar and Uttar Pradesh, 12.9--28.4 nonpolio AFP cases per 100,000 were identified during this period. Nationally, adequate stool specimens were collected from 84% of AFP cases during 2007--2008, and 86% of AFP cases from January through May 2009.
Stool specimens from AFP cases undergo virologic testing in one of the eight WHO-accredited national Global Polio Laboratory Network laboratories.** The national reference laboratory in Mumbai performs genomic sequence analysis of all WPV isolates.
WPV Epidemiology
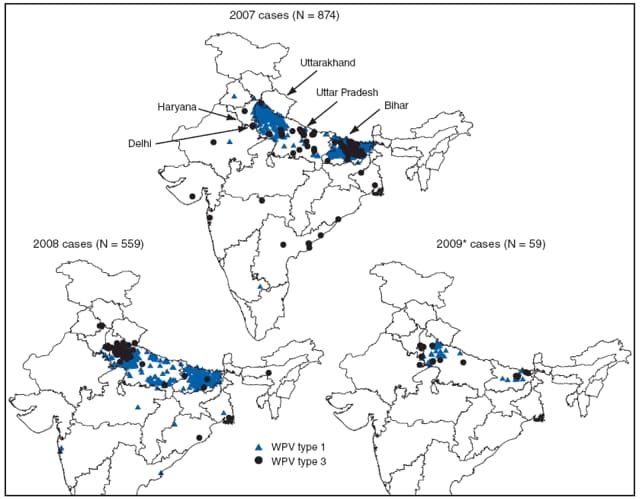
A total of 874 WPV cases were reported from 13 states in 2007 and 559 WPV cases were reported in 13 states in 2008 (Figures 2 and 3). During January--May 2009, 59 WPV cases were reported from four states; 279 cases were reported during the same period in 2008. Among cases reported during 2007--2008, 867 (61%) occurred in children aged <24 months and 44 (3%) occurred in children aged >5 years. Among cases reported during 2007--2008, 1,108 (77%) of the children received >7 doses of OPV, 265 (18%) received 4--7 doses, 40 (3%) received 1--3 doses, and 20 (1%) received zero doses or the number of doses received was unknown.
WPV1. A total of 83 WPV1 cases were reported in 45 districts in 2007, including 46 (55%) cases in Bihar and 22 (27%) in Uttar Pradesh. In 2008, 75 WPV1 cases were reported in 22 districts; among those cases, three (4%) were identified in two districts in Bihar and 62 (83%) were identified in 13 districts in an outbreak in Uttar Pradesh (which included 50 cases in five districts within western Uttar Pradesh). During January--May 2009, India reported 18 WPV1 cases in 11 districts; six cases (33%) were reported from two districts in Bihar and eight (44%) from seven districts in Uttar Pradesh.
The current outbreak in Uttar Pradesh, totaling 70 cases to date, began when a WPV1 case genetically linked to WPV circulating in Bihar was detected in western Uttar Pradesh in May 2008. Until then, no WPV1 cases had been reported in Uttar Pradesh since November 2007, and in the previously highest-risk districts of western Uttar Pradesh since September 2006. All 70 WPV1 cases in Uttar Pradesh are linked genetically to this introduction, and in November 2008; a case genetically linked to this outbreak was conversely detected in Bihar. Among the nine WPV1 cases in Bihar in 2008--2009 to date, at least two genetically distinct chains of transmission were identified, primarily localized in difficult-to-reach populations in the flood-prone areas of the Kosi River basin.
WPV3. In all of India, 794 WPV3 cases were reported in 78 districts in 2007 and 484 cases were reported in 85 districts in 2008; 779 (98%) and 473 (98%) of cases in 2007 and 2008, respectively, occurred in Bihar and Uttar Pradesh. During January--May 2009, 41 WPV3 cases were reported, versus 274 WPV3 cases reported during the same period in 2008. All 41 cases reported in 2009 have been in Bihar and Uttar Pradesh.
Reported by: Ministry of Health and Family Welfare, Government of India; National Polio Surveillance Project, WHO, New Delhi; Immunization and Vaccine Development Dept, WHO Regional Office for South-East Asia, New Delhi; UNICEF, New Delhi; Poliovirus Laboratory Network, Ahmedabad, Bangalore, Chennai, Coonoor, Kasauli, Kolkata, Lucknow, and Mumbai, India. Polio Eradication Dept, WHO, Geneva, Switzerland. Div of Viral Diseases and Global Immunization Div, National Center for Immunization and Respiratory Diseases; SE Kidd, MD, EIS Officer, CDC.
Editorial Note:
Overall, WPV1 incidence in India declined during 2007--2009 following implementation of the recommendations of the Global Advisory Committee on Polio Eradication and the India Expert Advisory Group for Polio Eradication to prioritize the elimination of WPV1; this involved conducting SIA rounds using mOPV1 as often as every 6--8 weeks in high-risk areas. In contrast to other polio-endemic countries, WPV transmission in the northern Indian states of western Uttar Pradesh and Bihar persists despite ≥95% of WPV cases reporting receipt of at least four OPV doses. Persistent transmission in these areas despite high vaccination coverage has been attributed to relatively lower vaccine effectiveness of OPV in northern India than in other populations, possibly resulting from a combination of a high incidence of diarrheal diseases, malnutrition, and a high force of WPV infection†† resulting from crowding (1,7,8). Among WPV case children, a very high proportion are vaccinated rather than unvaccinated, which reflects the frequency and high coverage of polio vaccination campaigns.
The interruption of WPV1 transmission in Uttar Pradesh during 2007--2008 indicates that frequent mOPV1 rounds of consistently high coverage with enhanced technical support can be successful, even in areas with the most persistent transmission. Western Uttar Pradesh districts have high population density, poor sanitation, and low socioeconomic status and have been the main reservoir for WPV1 transmission in India in previous years. The 2008--2009 Uttar Pradesh outbreak, caused by WPV1 introduced from Bihar, appears to be diminishing, although the risk for continued transmission or reintroduction persists. In Bihar, the intense focus on vaccinating populations in the Kosi River area has resulted in only three WPV1 cases being reported in 2008 and six cases through May 2009, despite severe floods in Bihar in 2008; these floods displaced high-risk populations, worsened sanitary conditions, and interfered with scheduled SIAs. Genetic data from WPV isolated from cases indicate that low-grade WPV1 transmission has continued during 2008--2009 in Bihar in districts of the Kosi River basin. This transmission, often undetected for several months, has resulted occasionally in WPV1 cases in several other states.
The number of reported WPV3 cases in India has declined steadily since the peak of the 2007 outbreak. Most WPV3 cases in 2008 occurred in districts in Uttar Pradesh and Bihar in which less than three SIA rounds of mOPV3 had been administered during 2007. The mOPV3 rounds conducted at the end of 2007 and during 2008 appear to have substantially reduced WPV3 transmission and limited transmission to Bihar and Uttar Pradesh in 2009. Although multiple importations of WPV1 and WPV3 were detected in areas outside Uttar Pradesh and Bihar during 2007--2008, no outbreaks of polio occurred in those other areas, in which prompt and large scale mop-up vaccination rounds, higher vaccine effectiveness, higher routine vaccination coverage, and continued national SIAs have produced higher levels of immunity and lower risk for transmission.
India plans to conduct additional mOPV3 SIA rounds as needed to prevent further WPV3 outbreaks while continuing to use mOPV1 for most SIAs. Based on preliminary data from a clinical trial, the Advisory Committee on Polio Eradication and the India Expert Advisory Group for Polio Eradication have recommended the use of bivalent type 1 and type 3 OPV to substitute for mOPV3 in future SIAs when available. The recently developed bivalent formulation is anticipated to be licensed for use in India later in 2009.
Reaching the goal of polio eradication in India is dependent on ongoing efforts to interrupt remaining WPV transmission simultaneously in high-risk areas of western Uttar Pradesh and Bihar, first WPV1, then WPV3. Strategies to accomplish that include reaching all children during SIAs in the Kosi River area and improving the effectiveness of polio vaccines. Potential interventions to improve the effectiveness of poliovirus vaccines under investigation include the use of inactivated poliovirus vaccine as a supplement to OPV, high-titer mOPV1, and zinc supplementation (9). Surveillance also has been expanded in high-risk areas to examine the potential contribution of older age groups to poliovirus transmission to inform a possible expansion of the target age group in these areas. Continued vigilance, sustained commitment, ongoing research and aggressive responses to new cases will be required to interrupt remaining WPV1 transmission and to eliminate polio in India. Polio eradication activities in India have provided successful operational models for elimination of transmission in many other areas of the world. Elimination of WPV circulation in India would further serve as a stimulus for the remaining countries with WPV transmission, and ultimately lead to global eradication.
References
- CDC. Progress toward interruption of wild poliovirus transmission---worldwide, 2008. MMWR 2009;58:308--12.
- CDC. Progress toward poliomyelitis eradication---India, January 2006--September 2007. MMWR 2007;56:1187--91.
- Grassly NC, Wenger J, Durrani S, et al. Protective efficacy of a monovalent oral type 1 poliovirus vaccine: a case-control study. Lancet 2007;369:1356--62.
- Government of India Ministry of Health and Family Welfare. District level household and facility survey (DLHS-3)---2007--2008 (provisional). Available at http://nrhm-mis.nic.in/ui/reports/dlhsiii/india%20fact%20sheet%20jun_final1.pdf.
- Government of India Ministry of Health and Family Welfare. District level household and facility survey (DLHS-3)---2007--2008. Available at http://www.rchiips.org/state-fact-sheet-rch3.html.
- CDC. Laboratory surveillance for wild and vaccine-derived polioviruses---worldwide, January 2007--June 2008. MMWR 2008;57:967--70.
- Grassly NC, Fraser C, Wenger J, et al. New strategies for the elimination of polio from India. Science 2006;314:1150--3.
- Grassly NC, Wenger J, Durrani S, et al. Protective efficacy of a monovalent oral type 1 poliovirus vaccine: a case-control study. Lancet 2007;369:1356--62.
- Zinc Investigators Collaborative Group. Prevention of diarrhea and pneumonia by zinc supplementation in children in developing countries: pooled analysis of randomized controlled trials. J Pediatr 1999;135:689--97.