 |
|
|
|
|
|
|
| ||||||||||
|
|
|
|
|
|
|
||||
| ||||||||||
|
|
|
|
|
Persons using assistive technology might not be able to fully access information in this file. For assistance, please send e-mail to: mmwrq@cdc.gov. Type 508 Accommodation and the title of the report in the subject line of e-mail. Rift Valley Fever Outbreak --- Kenya, November 2006--January 2007In mid-December 2006, several unexplained fatalities associated with fever and generalized bleeding were reported to the Kenya Ministry of Health (KMOH) from Garissa District in North Eastern Province (NEP). By December 20, a total of 11 deaths had been reported. Of serum samples collected from the first 19 patients, Rift Valley fever (RVF) virus RNA or immunoglobulin M (IgM) antibodies against RVF virus were found in samples from 10 patients; all serum specimens were negative for yellow fever, Ebola, Crimean-Congo hemorrhagic fever, and dengue viruses. The outbreak was confirmed by isolation of RVF virus from six of the specimens. Humans can be infected with RVF virus from bites of mosquitoes or other arthropod vectors that have fed on animals infected with RVF virus, or through contact with viremic animals, particularly livestock. Reports of livestock deaths and unexplained animal abortions in NEP provided further evidence of an RVF outbreak. On December 20, an investigation was launched by KMOH, the Kenya Field Epidemiology and Laboratory Training Program (FELTP), the Kenya Medical Research Institute (KEMRI), the Walter Reed Project of the U.S. Army Medical Research Unit, CDC-Kenya's Global Disease Detection Center, and other partners, including the World Health Organization (WHO) and Médecins Sans Frontières (MSF). This report describes the findings from that initial investigation and the control measures taken in response to the RVF outbreak, which spread to multiple additional provinces and districts, resulting in 404 cases with 118 deaths as of January 25, 2007. Teams of investigators conducted patient interviews and reviewed medical records from December 1 forward in major health-care facilities in the districts from which cases were first reported. The teams detected additional cases by meeting with elders, other leaders, and health-care providers in villages where cases had been reported and in adjacent villages. Blood samples from patients with suspected RVF were collected and maintained at 39.2ºF (4.0ºC). Samples from NEP and surrounding areas were transported to a field laboratory established at Garissa Provincial Hospital by CDC, KEMRI, and KMOH; samples from other areas were sent to KEMRI laboratories in Nairobi and to a laboratory in Malindi that was supported by a team from Health Canada. A suspected case was defined as acute onset of fever (>99.5ºF [>37.5ºC]) with headache or muscle and joint pain since December 1 in a person who had no other known cause of acute febrile illness (e.g., malaria). A probable case was defined as acute onset of fever in a person with unexplained bleeding (i.e., in stool, vomit, or sputum or from gums, nose, vagina, skin, or eyes), vision deterioration, or altered consciousness. A confirmed case was defined as a suspected or probable case with laboratory confirmation of the presence in serum of anti-RVF virus IgM by enzyme-linked immunosorbent assay (ELISA) or RVF virus RNA by reverse transcription--polymerase chain reaction (RT-PCR). The index case was reported in Garissa District in a patient who had symptom onset on November 30, 2006. Retrospective analysis of sera collected during July--November 2006 at Garissa Provincial Hospital revealed no evidence of earlier acute RVF infections. As of January 25, 2007, a total of 404 cases of RVF had been reported in Kenya with 118 deaths, a case-fatality rate of 29%. Of the reported cases, 115 (29%) were laboratory confirmed by anti-RVF virus IgM by ELISA (64 cases, 56%) or RT-PCR (79, 69%), including 28 cases (24%) confirmed by both. Of the remaining 289 cases, 109 were classified as probable. Of the 230 patients with available demographic information, 140 (61%) were male (Figure 1). Patients ranged in age from 4 to 85 years, with a median age of 27 years (30 years for females and 25 years for males). RVF cases were reported from three districts in NEP (Garissa [175 cases], Ijara [125], and Wajir [26]); five districts in Coast Province (Kilifi [38], Tana River [16], Malindi [eight], Isiolo [eight], and Taita Taveta [one]); two districts in Central Province (Kirinyanga [two] and Maragua [one]); one district in Rift Valley Province (Kajiado [three]); and one from Nairobi Area (Figure 2). The patient from Nairobi had traveled to NEP during the week before illness onset but was hospitalized in Nairobi. Ijara (population 79,932) and Garissa (population 420,918) districts had the highest RVF incidence rates: 156 and 42 per 100,000 population, respectively. Among the first 97 reported cases from Garissa and Wajir districts with detailed epidemiologic information available, 71 (73%) met the probable case definition; 38 of the 62 patients who provided blood samples tested positive by IgM ELISA, RT-PCR, or both. The most frequently reported symptoms among the 97 patients were fever (100%), headache (90%), bleeding (76%), malaise (70%), muscle pain (62%), back pain (60%), vomiting (56%), and joint pain (51%). Two thirds of the 66 patients who provided information on potential risk factors reported having an animal that was recently ill. The most frequently reported RVF risk factors during the 2 weeks preceding illness onset were drinking unboiled (raw) milk (72%); living within 100 meters of a swamp (70%); having an ill animal (67%); drinking milk from an ill animal (59%); working as a herdsman (50%); having a dead animal (50%); and slaughtering an animal (42%). Approximately 9% of patients reported contact with another ill human. The outbreak peaked on December 24, 2006, and the number of daily cases has been declining since December 27, 2006 (Figure 3). A ban on livestock slaughtering in Garissa District went into effect on December 27 and was expanded as RVF was detected in additional districts. Vaccination of animals with live, attenuated RVF vaccine began on January 8, 2007. Prevention messages were developed in three languages (English, Kiswhali, and Somali), and public meetings (known as barazas) were held to spread information rapidly to the community. Messages also were disseminated via radio, a widely used communication medium in NEP. Village elders, chiefs, and religious leaders were consulted throughout Garissa District, leading to a district ban on the slaughter of livestock and closure of the livestock market. Health-care workers were trained to care for persons suspected to be infected with RVF virus. Reported by: P Nguku, S Sharif, A Omar, C Nzioka, P Muthoka, J Njau, A Dahiye, T Galgalo, J Mwihia, J Njoroge, H Limo, J Mutiso, R Kalani, A Sheikh, J Nyikal, Kenya Ministry of Health; D Mutonga, J Omollo, A Guracha, J Muindi, S Amwayi, D Langat, D Owiti, A Mohammed, Kenya Field Epidemiology and Laboratory Training Program, Kenya Ministry of Health/CDC. J Musaa, Kenya Ministry of Livestock and Fisheries Development. R Sang, Kenya Medical Research Institute. R Breiman, K Njenga, D Feikin, M Katz, H Burke, P Nyaga, M Ackers, S Gikundi, V Omballa, L Nderitu, N Wamola, R Wanjala, S Omulo, International Emerging Infections Program, Global Disease Detection Center, CDC Kenya. J Richardson, D Schnabel, S Martin, US Army Medical Research Unit-Kenya. D Hoel, H Hanafi, M Weiner, US Naval Medical Research Unit No. 3, Cairo, Egypt. J Onsongo, T Kojo, M Duale, A Hassan, M Dabaar, C Njuguna, Kenya Country Office; M Yao, African Regional Office; T Grein, P Formenty, World Health Organization, Geneva, Switzerland. B Telfer, Médecins Sans Frontières Belgium; R Lepec, Médecins Sans Frontières Epicentre, Paris, France. H Feldmann, A Grolla, Health Canada, Winnipeg, Manitoba. S Wainwright, Animal and Plant Health Inspection Svc, US Dept of Agriculture. Global Disease Detection Program, Coordinating Office for Global Health; Div of Global Migration and Quarantine, Div of Emerging Infections and Surveillance Services, National Center for Preparedness, Detection, and Control of Infectious Diseases (proposed); Special Pathogens Br, Div of Viral and Rickettisial Diseases, Div of Vector-Borne Infectious Diseases, National Center for Zoonotic, Vector-Borne and Enteric Diseases (proposed); E Lederman, E Farnon, C Rao, BK Kapella, H Gould, EIS officers, CDC. Editorial Note:RVF is an acute, febrile zoonotic disease caused by Rift Valley fever virus, which belongs to the family Bunyaviridae and genus Phlebovirus. The virus is primarily a vector-borne zoonotic pathogen. Humans acquire RVF through bites from infected mosquitoes or, more frequently, through exposure to the blood, body fluids, or tissues of animals that have been bitten by infected mosquitoes. Direct exposure to infected animals can occur during slaughter or through veterinary and obstetric procedures. RVF was first described in sheep in the early 20th century, and the virus was first isolated in humans in Kenya in 1930 (1,2). In livestock, RVF causes abortion and death. Livestock epizootics can occur after heavy rainfall and flooding that result in hatching of Aedes mosquitoes (thought to be the initial vector and inter-epizootic reservoir of RVF) and other vectors that feed on nearby mammals (3). Eastern Kenya experienced unusually heavy rainfall during October--December 2006, three times the average for that period during the preceding 8 years and 13 times the rainfall in 2005 (Kenya Meteorological Department, unpublished data, 2007). Patients with RVF usually have initial signs and symptoms of influenza-like illness; less than 8% of patients subsequently have severe disease, including generalized hemorrhagic syndrome, encephalitis, or retinitis (2). The overall human mortality rate from RVF has been estimated at 0.5%--1.0% of those infected, but the rate is much higher among those with severe disease. The largest reported human outbreak occurred in Kenya during 1997--1998, in which an estimated 89,000 persons (based on a systematic serosurvey) were infected and 478 died; this outbreak also was centered in NEP (3--5). Previous RVF outbreaks among humans were not reported outside sub-Saharan Africa until 1977--1978, when approximately 18,000 persons became ill with RVF in Egypt, and in 2000, when approximately 800 persons in Saudi Arabia and 1,000 in Yemen had severe illness (6--8). Like the 1997--1998 outbreak, the current outbreak was associated with heavy rainfall, which produced massive flooding in much of Kenya, and particularly in NEP. Climatic forecasting in conjunction with satellite imaging of flooded areas has been suggested as a method for predicting where and when RVF outbreaks might occur, potentially enabling earlier interventions (9). Most of the cases before December 20 occurred in young men who herded livestock, perhaps because herdsmen are the first to identify and slaughter ill animals. Later in the outbreak, the distribution of cases broadened by age and sex. Young women also were overrepresented, perhaps because they handle uncooked animal products at home as they prepare meals for the family. Cases among children aged <5 years and the elderly have been rare, probably because they rarely interact with animals or handle raw animal products. Most patients reported to KMOH had severe illness with bleeding, which likely accounts for the 29% case-fatality rate. Judging from previous studies, many mild, undetected RVF virus infections likely occurred during this outbreak (5). Additional cases of severe disease also might have occurred in NEP but were not detected because of the inaccessibility of many areas of the province resulting from flooding. Many areas of NEP, including an entire division of Garissa District, were unreachable by road from early December to mid-January. Since mid-January, RVF in livestock has been detected in districts surrounding Nairobi, signaling occurrence of the outbreak in new areas. Reports also have been received of livestock and humans with illness consistent with RVF across the border in Somalia, where disease assessment has been hampered by ongoing security concerns. Several international organizations are collaborating to control the spread of the outbreak within Kenya and to other countries. Travelers should take precautions when visiting RVF-affected areas. Generally, the risk for RVF infection among travelers to Kenya is low, unless they visit areas where an outbreak is occurring and are bitten by infected mosquitoes or come in contact with body fluids, uncooked tissue, or aerosols from infected livestock. No preventive RVF medications or licensed vaccines for humans exist. Travelers to affected areas should reduce their risk for infection by protecting themselves from mosquito bites and by avoiding direct contact with livestock. Specific recommendations for U.S. travelers are available at http://www.cdc.gov/travel/other/2006/rift_valley_fever_kenya.htm. To control the outbreak, KMOH launched several interventions, some of which might have limited the public health impact of the outbreak. A ban on the slaughter of animals (including during Eid-ul-azha, a religious holiday) was imposed in NEP and strictly enforced. The Ministry of Livestock and Fisheries Development initiated a policy of vaccinating apparently unaffected herds of livestock in districts in which human or livestock RVF disease had been confirmed and also in adjacent districts; however, as of January 25, only a small proportion of livestock had been immunized. Other interventions included heightened disease surveillance among humans and animals, community mobilization, animal quarantines and restricted transport of livestock, and an integrated vector-control strategy, including indoor residual spraying and larviciding. RVF wards were established in which appropriate infection-control measures were encouraged. Timely detection of this outbreak was aided by implementation of Integrated Disease Surveillance and Response* within most of the affected districts. A second factor contributing to timely detection was initiation of RVF laboratory-supported field surveillance of febrile patients at outpatient clinics in Garissa. Ongoing epidemiologic, entomologic, and veterinary studies related to this outbreak continue to 1) identify factors associated with severe forms of RVF illness and poor outcomes; 2) characterize the role of specific species of mosquitoes in transmitting, maintaining, and spreading RVF virus; 3) assess the economic impact of the outbreak; and 4) define the impact of livestock immunization with live, attenuated RVF veterinary vaccine on minimizing the spread of animal and human disease. Taking measures to decrease contact with mosquitoes through use of repellents and bednets and avoiding exposure to blood or tissues of animals that might be infected are important protective measures for preventing RVF. Livestock vaccination also can be an effective means of preventing cases of human RVF if adequate vaccination coverage and herd immunity are achieved. Acknowledgments This report is based, in part, on contributions by S Konongoi, V Ofula, J Lutomiah, C Ochieng, M Warigia, Kenya Medical Research Institute, and R Lindsay, Health Canada, Winnipeg, Manitoba. References
* A strategy of the African Regional Office of WHO that aims to improve availability and use of surveillance and laboratory data to control infectious diseases that are the leading causes of death, disability, and illness in the region.
Figure 1 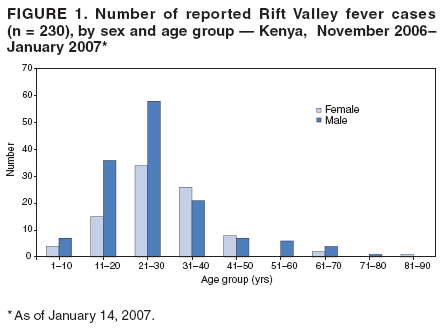 Return to top. Figure 2 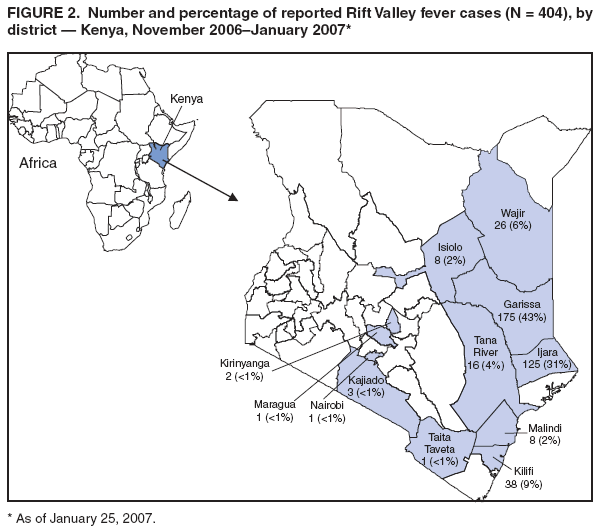 Return to top. Figure 3 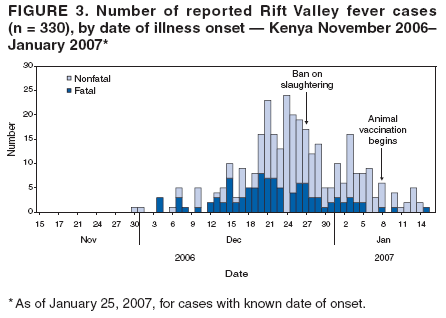 Return to top.
Disclaimer All MMWR HTML versions of articles are electronic conversions from ASCII text into HTML. This conversion may have resulted in character translation or format errors in the HTML version. Users should not rely on this HTML document, but are referred to the electronic PDF version and/or the original MMWR paper copy for the official text, figures, and tables. An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S. Government Printing Office (GPO), Washington, DC 20402-9371; telephone: (202) 512-1800. Contact GPO for current prices. **Questions or messages regarding errors in formatting should be addressed to mmwrq@cdc.gov.Date last reviewed: 1/31/2007 |
|||||||||
|