 |
|
|
|
|
|
|
| ||||||||||
|
|
|
|
|
|
|
||||
| ||||||||||
|
|
|
|
|
Persons using assistive technology might not be able to fully access information in this file. For assistance, please send e-mail to: mmwrq@cdc.gov. Type 508 Accommodation and the title of the report in the subject line of e-mail. Prevalence of IgG Antibody to SARS-Associated Coronavirus in Animal Traders --- Guangdong Province, China, 2003Severe acute respiratory syndrome (SARS) was identified in 2003 as an infectious disease caused by the SARS-associated coronavirus (SARS-CoV), a member of the coronavirus family not observed previously in humans (1,2). Because its sequence data differ from that of known human coronaviruses, SARS-CoV is suspected to have crossed the species barrier between an animal host and humans. The SARS outbreak began in China's Guangdong Province, where approximately 1,500 probable cases were identified during November 2002--June 2003 (3). Detection of SARS-like coronavirus has been reported previously in masked palm civets (sometimes called civet cats) and a raccoon dog for sale in a live animal market in Shenzhen municipality (4). This report summarizes results of an investigation conducted by public health authorities in Guangdong Province, which compared the seroprevalence of SARS-CoV IgG antibody in animal traders (i.e., workers in live animal markets) with that of persons in control groups. The results indicated that 13% of the animal traders, none of whom had SARS diagnosed, had IgG antibody to SARS-CoV, compared with 1%--3% of persons in three control groups. Although the results provide indirect support for the hypothesis of an animal origin for SARS, they also underscore the need for detailed patient histories and more focused animal studies to confirm an animal origin for SARS. The seroprevalence study was conducted by the Guangdong Center for Disease Control and Prevention (CDC) in conjunction with the Guangzhou CDC, Baiyun District CDC, and Shijing Township Hospital. Traders in three animal markets in Guangzhou, Guangdong Province, were offered participation in the study, and samples were collected on May 4, 2003, from those who gave consent. The trader test results were compared with those for persons in three control groups: 1) health-care workers involved with SARS control in two city hospitals, 2) public health workers in the Guangdong CDC facility, and 3) healthy adults visiting a clinic for routine physical examinations. Compared with the overall control population, the animal traders were more likely to be male and older; the majority of persons in both the trader and control groups were aged 20--39 years. A sample of blood (5 mL) was drawn from each subject, and IgG antibody to SARS-CoV was tested by enzyme-linked immunosorbent assay (ELISA) by using the test kit (batch no. 20030501) manufactured by Beijing Huada GBI Biotechnology Co. Ltd., Beijing. Of 792 persons tested, IgG antibody to SARS-CoV was detected in 72 (9.1%). Positive rates were highest in the trader group (13.0%), compared with the three control groups (range: 1.2%--2.9%) (Table 1). The prevalence of IgG antibody in the trader group was statistically significantly higher than that of the overall control population (chi square = 26.1; p<0.01). In contrast, no statistically significant difference was determined in the prevalence of antibody detected among the three control groups (chi square = 0.89; p = 0.64). Among animal traders, the highest prevalence of antibody was found among those who traded primarily masked palm civets (72.7%), wild boars (57.1%), muntjac deer (56.3%), hares (46.2%), and pheasant (33.3%) (Table 2). The prevalence of traders with IgG antibody to SARS-CoV varied by market (6%, 11%, and 20%, respectively; p<0.001); no correlation was found between SARS-CoV antibody and sex, age, or number of years worked in a live animal market. None of the subjects had SARS or atypical pneumonia diagnosed during the Guangdong Province outbreak. Reported by: D Yu, MD, H Li, R Xu, MPH, J He, J Lin, L Li, W Li, H Xu, S Huang, J Huang, Guangdong Center for Disease Control, Guangzhou, China. Editorial Note:This study found serologic evidence suggesting that asymptomatic infection with SARS-CoV or an antigenically related virus occurred in Guangdong Province. Seroprevalence of IgG antibody to SARS-CoV was substantially higher among traders of live animals than among persons in control groups, consistent with the hypothesis that SARS-CoV crossed the species barrier from animals to humans. The results are consistent with preliminary determinations of a joint research team from China's Ministry of Agriculture and Guangdong Province, which found that sequences of coronavirus detected by polymerase chain reaction in bats, monkeys, masked palm civets, and snakes were identical to or similar to those of human SARS-CoV isolates. In addition, a joint study by Shenzhen CDC and Hong Kong University determined that the sequence of coronavirus isolated from masked palm civets is 99% identical to human SARS-CoV (4). These determinations appear consistent with the hypothesis that an animal reservoir exists for SARS-CoV or an antigenically related virus; however, the findings are not sufficient to identify either the natural reservoir for SARS-CoV or the animal(s) responsible for crossover to humans. Primary modes of SARS transmission probably are direct contact or droplet spread from a patient symptomatic with SARS; however, other routes of transmission might exist (5). Approximately 63% of Guangdong Province patients with clinically defined SARS had no known history of exposure to other SARS patients, and the percentage increased after April 2003 (6). This trend of unknown exposure also was observed in other areas (7). Therefore, the possibility of unrecognized sources of infection or infection from asymptomatic carriers of the virus cannot be excluded, although some patients might also have pneumonia caused by etiologies other than SARS-CoV. The findings in this report are subject to at least four limitations. First, although subjects were categorized as primarily traders of the animals they were selling at the time of the survey, a substantial portion traded or handled more than one type of animal. Second, the small number of subjects with reported exposure to certain types of animals limits the ability to differentiate risk among specific groups of animal traders. Third, although the animal traders worked at three markets in Guangzhou, risk might differ among traders in other parts of Guangdong Province or elsewhere in China. Finally, as with other urgently developed tests, validation of the ELISA kit employed has not been completed, and the IgG antibody cannot distinguish recent from remote infection. This report provides indirect support for the hypothesis that SARS-CoV might have originated from an animal source and identifies multiple animals for further study. However, none of the traders in this study had SARS, and only two SARS patients in Guangdong Province were identified as animal traders (i.e., a snake seller and a pigeon seller) (6). In contrast, comparative analysis of early Guangdong cases, unlinked to other SARS cases, indicated an overrepresentation of food handlers (6). Whether the antibody detected in the animal traders in this report might represent infection with a related coronavirus that cross-reacts with SARS-CoV, or whether that antibody provides protection from SARS, is not known. Efforts to identify a possible animal reservoir for SARS might benefit from prompt attention to collecting detailed histories from any future SARS patients regarding animal and other environmental exposures and initiating tracebacks to animal supply sources (e.g., markets, farms, and wildlife areas). Acknowledgments This report was based on contributions by Guangzhou Municipal Center for Disease Control (CDC), Baiyun District CDC; Shijing Township Hospital, Guangdong Province; CK Lee, MD, World Health Organization (WHO)--China SARS Team, Beijing, China. A Schuchat, MD, WHO-China SARS Team and National Center for Infectious Diseases, CDC. References
Table 1 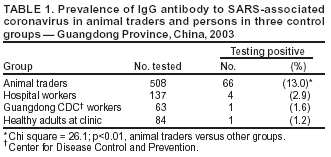 Return to top. Table 2 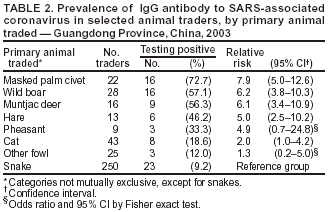 Return to top.
Disclaimer All MMWR HTML versions of articles are electronic conversions from ASCII text into HTML. This conversion may have resulted in character translation or format errors in the HTML version. Users should not rely on this HTML document, but are referred to the electronic PDF version and/or the original MMWR paper copy for the official text, figures, and tables. An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S. Government Printing Office (GPO), Washington, DC 20402-9371; telephone: (202) 512-1800. Contact GPO for current prices. **Questions or messages regarding errors in formatting should be addressed to mmwrq@cdc.gov.Page converted: 10/16/2003 |
|||||||||
This page last reviewed 10/16/2003
|