COVID-19 Science Update released: December 10, 2021 Edition 116

The COVID-19 Science Update summarizes new and emerging scientific data for public health professionals to meet the challenges of this fast-moving pandemic. Weekly, staff from the CDC COVID-19 Response and the CDC Library systematically review literature in the WHO COVID-19 database, and select publications and preprints for public health priority topics in the CDC Science Agenda for COVID-19 and CDC COVID-19 Response Health Equity Strategy.
Section headings in the COVID-19 Science Update have been changed to align
with the CDC Science Agenda for COVID-19.
Here you can find all previous COVID-19 Science Updates.
PREPRINTS (NOT PEER-REVIEWED)
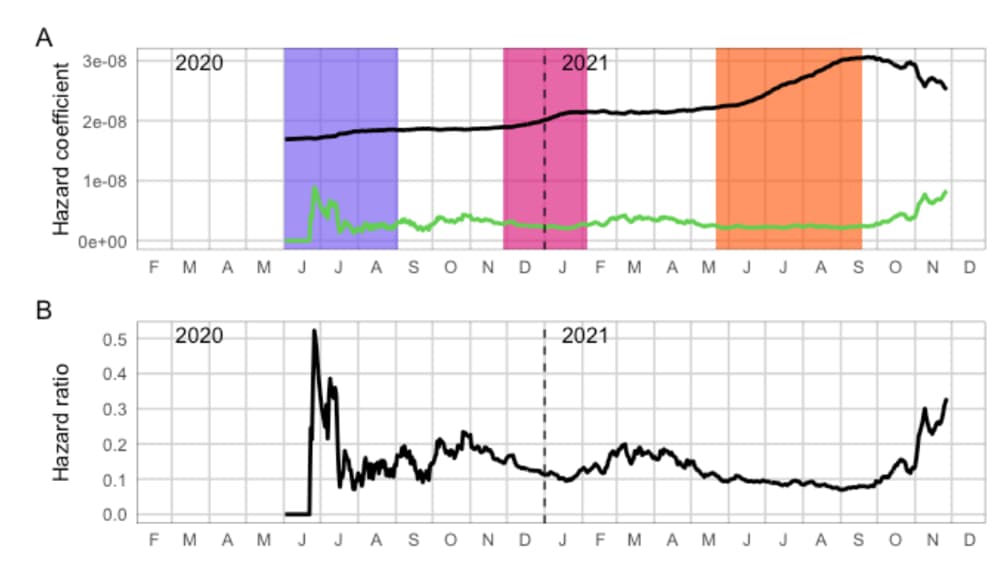
Increased risk of SARS-CoV-2 reinfection associated with emergence of the Omicron variant in South Africa. Pulliam et al. medRxiv (December 2, 2021). Published in Science (March 15, 2022).
Key findings:
- Compared with the 1st SARS-CoV-2 wave, the estimated ratio of reinfection hazard to primary infection hazard was lower during wave 2 (Beta [1.351]) (hazard ratio [HR] 0.75, 95% CI 0.59-0.97) and wave 3 (Delta [B.167.2]) (HR 0.71, 95% CI 0.56-0.92), but higher during November 1–27, 2021 (HR 2.39, 95% CI 1.88-3.11), coincident with the emergence of the Omicron (B.1.1.529) variant (Figure).
Methods: Retrospective study of reinfection among 2,796,982 persons in South Africa with laboratory-confirmed positive SARS-CoV-2 tests collected through a national surveillance system from March 4, 2020–November 27, 2021. Risk of primary infection, reinfection (sequential positive RT-PCR or antigen tests ≥ 90 days apart), and relative hazard of reinfection vs. primary infection were calculated. Limitations: Vaccination status of reinfected individuals was not known; detection rates may vary across time and by characteristics related to access to testing; hazard ratio only uses 2nd infection as reinfections; model does not account for potential of waning immunity; antigen testing may be under-reported in the data set.
Implications: The Omicron variant appears to increase the risk of reinfection, suggesting it might have increased ability to evade immunity from prior infection. More research is needed to determine if similar results are seen for vaccine-induced immunity.
Figure:
PEER-REVIEWED
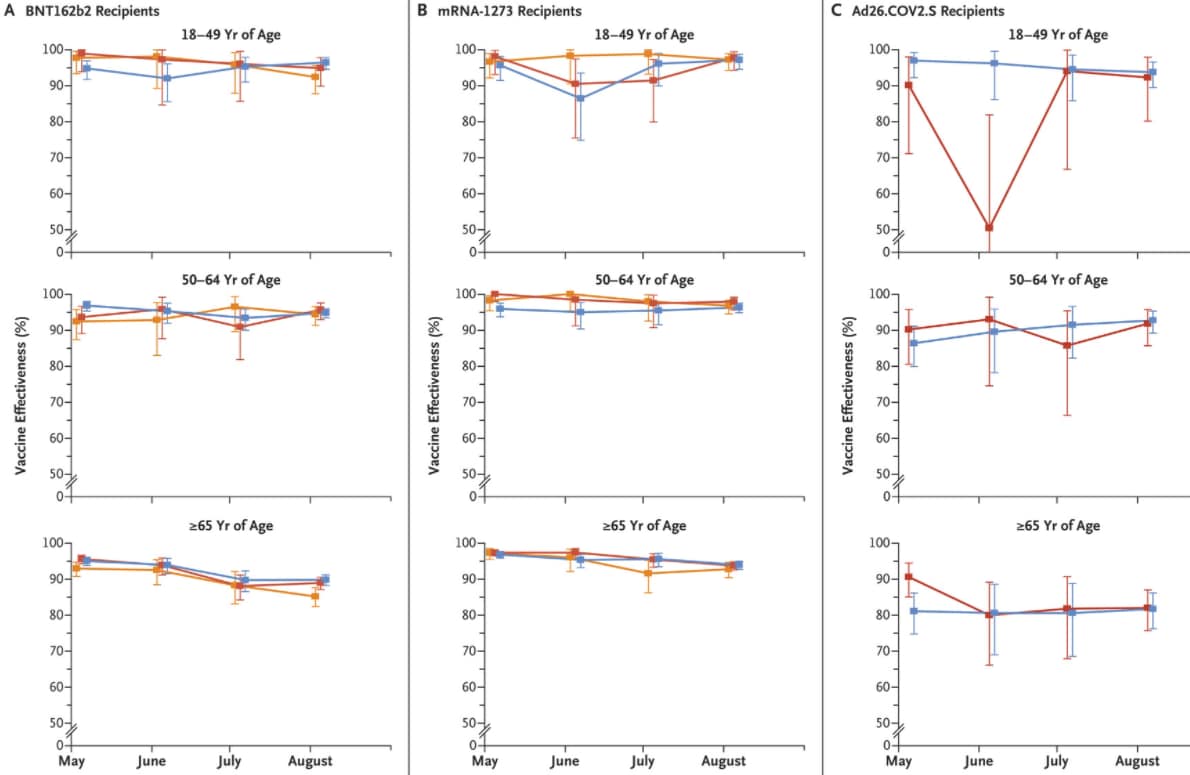
COVID-19 vaccine effectiveness in New York State. Rosenberg et al. NEJM (December 1, 2021).
Key findings:
- Vaccine effectiveness (VE) against SARS-CoV-2 infection declined from 93.4% in May to 74.2% by the end of August 2021.
- VE against hospitalization generally remained high for adults of all ages (Figure):
- Among adults aged 18–64 years, VE against hospitalization exceeded 86%.
- Among adults aged >65 years, VE against hospitalization was higher for recipients of BNT162b2 (Comirnaty, Pfizer/BioNTech) and mRNA-1273 (Moderna) compared with COV2.S (Johnson & Johnson/Janssen) but declined over time; VE among recipients of Ad26.COV2.S did not decline over time.
Methods: Prospective cohort study of New York state residents (n = 8,690,825), aged ≥18 years; weekly hazard rates were estimated by vaccine, product, age, and timing of vaccination cohort for VE against laboratory-confirmed SARS-CoV-2 infection (May 1–September 3, 2021) and COVID-19 hospitalization (May 1–August 31, 2021). Limitations: Indirect effects (e.g., herd immunity) and effect of prior SARS-CoV-2 infections were not assessed.
Implications: COVID-19 VE against hospitalization remains high despite the emergence of the Delta (B.1.617.2) variant, but declining VE among adults ≥65 years of age underscores the importance of prioritizing this group for vaccine boosters.
Figure:
Note: Adapted from Rosenberg et al. Estimated vaccine effectiveness against COVID-19 hospitalization May–August 2021 by vaccine type (BNT162b2 [A], mRNA-1273 [B], Ad26.COV2.S [C]), age group, and time of full vaccination (January–February 2021, March 2021, April 2021). 𝙸 bars indicate 95% CIs. From New England Journal of Medicine, Rosenberg et al., COVID-19 vaccine effectiveness in New York State, December 1, 2021 online ahead of print. Copyright © 2021 Massachusetts Medical Society. Reprinted with permission from Massachusetts Medical Society.
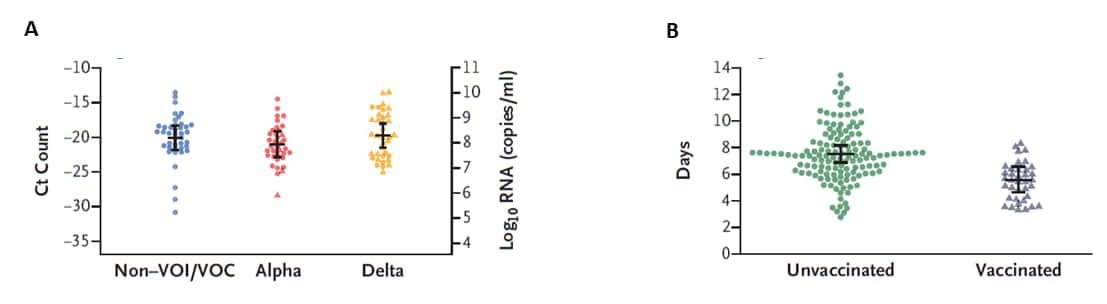
Viral dynamics of SARS-CoV-2 variants in vaccinated and unvaccinated. Kissler et al. NEJM (December 1, 2021).
Key findings:
- Among participants with SARS-CoV-2 infection, levels of viral RNA detected, proliferation duration, clearance duration, and duration of acute infection were similar among those infected with Alpha (B.1.1.7), Delta (B.1.617.2), and other variants not of current interest or concern (Non-VOI/VOC) (Figure).
- The proportion of participants with a peak Ct <15 (where lower peak Ct indicates higher levels of viral RNA detected) was slightly higher among those with Delta variant infections (13.0%) compared with Alpha variant (6.9%) or Non-VOI/VOC (10.2%) infections.
- Mean peak levels of viral RNA detected were similar between vaccinated and unvaccinated participants, but breakthrough infections in vaccinated persons “cleared” faster (mean 5.5 days, 95% credible interval 4.6-6.5) compared with unvaccinated persons (mean 7.5 days, 95% credible interval 6.8-8.2).
Methods: SARS-CoV-2 specimens (n = 19,941) obtained from 173 infected National Basketball Association participants from November 28, 2020–August 11, 2021. Viral dynamics among participants infected with Alpha (B.1.1.7 [n = 36]), Delta (B.1.617.2 [n = 36]), and non-VOI/VOC (n = 41), and among fully vaccinated (n = 37) and unvaccinated (n = 136) were compared using Bayesian hierarchical modeling. Limitations: Small sample size prevented sub-analysis; participants were mostly healthy young men; Ct values were used to determine viral genome equivalents thus indirectly measuring viral loads; metric used to determine clearance of acute infection was not defined; findings might not be generalizable.
Implications: In this study, fully vaccinated individuals had a shorter period (2-days less) from peak viremia to clearance of SARS-CoV-2 infection than unvaccinated persons. While additional research on differences in viral dynamics is needed, COVID-19 vaccination is recommended to reduce transmission of SARS-CoV-2 across all variants.
Figure:
Note: Adapted from Kissler et al. Peak viral load according to variant status (A) and viral clearance time according to vaccination status (B). Horizontal lines indicate means; 𝙸 bars indicate 95% credible intervals. Non-VOI/VOC = variants not of current interest or concern. From New England Journal of Medicine, Kissler et al., Viral dynamics of SARS-CoV-2 variants in vaccinated and unvaccinated, December 1, 2021 online ahead of print. Copyright © 2021 Massachusetts Medical Society. Reprinted with permission from Massachusetts Medical Society.
PREPRINTS (NOT PEER-REVIEWED)
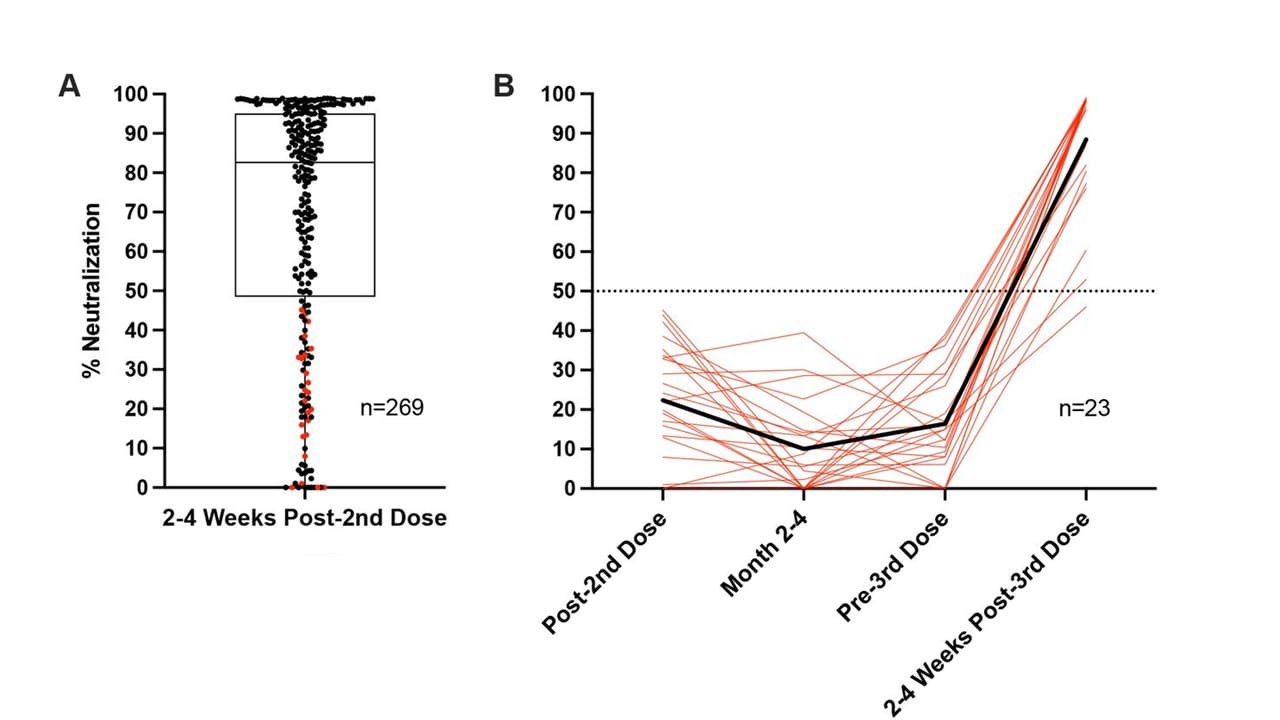
Third COVID-19 vaccine dose boosts neutralizing antibodies in poor responders. Lake et al. medRxiv (December 2, 2021).
Key findings:
- 2–4 weeks after a 2nd dose of mRNA COVID vaccine, 25% of adults had SARS-CoV-2 neutralizing antibody (NAb) capacity <50% to receptor binding domain (RBD) and were considered “vaccine poor responders” (VPRs) (Figure).
- 14% of 2-dose mRNA-1273 (Moderna) recipients were VPRs, compared with 31% of 2-dose BNT162b2 (Comirnaty, Pfizer/BioNTech) recipients.
- VPRs who received a 3rd vaccine dose showed an average 20-fold increase in NAb capacity (to average neutralization of 88%, range 49%-99%) 2–4 weeks post-3rd
Methods: SARS-CoV-2 NAb levels were measured in adults aged 18–80 years vaccinated with BNT162b2 (n = 180) or mRNA-1273 (n = 89). 23 VPRs received a 3rd vaccine dose 1–8 months (mean 5 months) after their 2nd dose. NAbs (reported as percent neutralization) were detected by lateral flow assay at 2–4 weeks and 2–4 months post-2nd dose, and at 1–2 weeks prior to 3rd dose and 2–4 weeks post-3rd dose. Limitations: Small sample size for VPRs; variability in administration of 3rd dose (manufacturer, timing); measured only antibodies to RBD that can be captured by ACE2 receptor on a lateral flow assay.
Implications: Persons fully vaccinated with an mRNA-based vaccine but with lower SARS-CoV-2 NAb capacity can elicit a robust immune response after a 3rd dose.
Figure:
Note: Adapted from Lake et al. A) Percent neutralization 2–4 weeks post-2nd dose. Horizontal line and box indicate median and interquartile range. Red dots indicate participants who received a 3rd dose. B) Percent neutralization in vaccine poor responders (VPRs) at 2–4 weeks post-2nd dose, 2–4 months post-2nd dose, 1–2 weeks pre-3rd dose, and 2–4 weeks post-3rd dose. Each red line indicates a single individual; black line indicates average. A 50% neutralization capacity roughly correlated to titer of 80 on a focus reduction neutralization test titer. Used by permission of authors.
PEER-REVIEWED
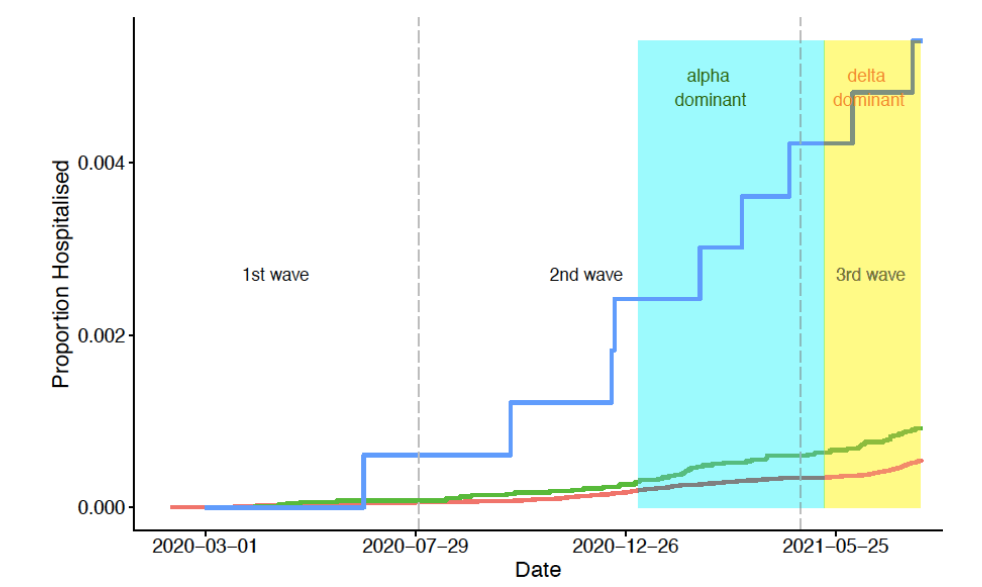
Risk of COVID-19 hospital admission among children aged 5–17 years with asthma in Scotland: A national incident cohort study. Shi et al. The Lancet Respiratory Medicine (November 30, 2021).
Key findings:
- Children with asthma had a higher risk of COVID-19 hospitalization compared with children without asthma (adjusted hazard ratio [aHR] 1.49, 95% CI 1.14-1.94).
- Children with poorly controlled asthma (aHR 6.40, 95% CI 3.27-12.53) and those with well-controlled asthma (aHR 1.36, 95% CI 1.02-1.80) had a greater risk of COVID-19 hospitalization compared with children with no asthma (Figure).
- A history of 2 or more corticosteroid prescriptions (another marker of asthma severity) was also associated with an elevated risk of COVID hospitalization compared with those with no asthma.
Methods: Analysis of linked data of children aged 5–17 years enrolled in the Early Pandemic Evaluation and Enhanced Surveillance of COVID-19 (EAVE II) cohort study in Scotland from March 2020–July 2021 (n = 752,867). Limitations: Small absolute numbers of COVID-19 hospital admissions; surrogate markers were used to classify poorly controlled asthma.
Implications: Children with asthma, especially those with recent history of asthma hospitalization or multiple courses of steroids for asthma exacerbations, are at elevated risk of hospitalization for COVID-19 and should be prioritized for COVID-19 vaccination and other prevention measures.
Figure:
Note: Adapted from Shi et al. COVID-19 hospitalization over time among children aged 5–17 years with poorly controlled asthma (asthma hospitalization in the 2 years prior to March 1, 2020), children with well controlled asthma (no asthma hospitalization in the 2 years prior to March 1, 2020) or children without asthma. The time period in the plots is marked by different waves (indicated by the dotted vertical lines); 1st wave from March to July 2020, 2nd wave from August 2020 to April 2021, and 3rd wave from May to July 2021. Licensed under CC-BY.
PREPRINTS (NOT PEER-REVIEWED)
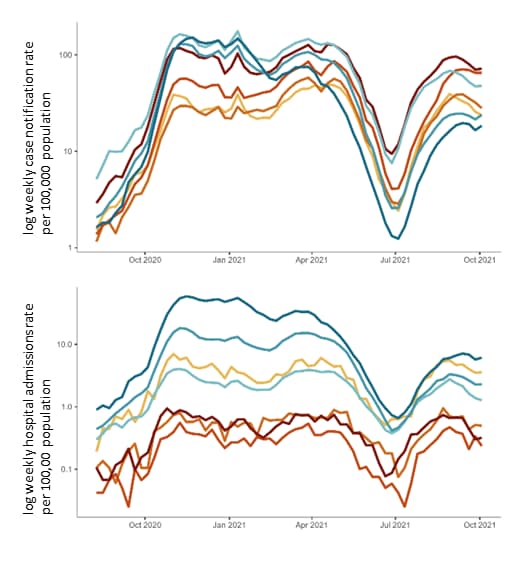
COVID-19 trends and severity among symptomatic children aged 0-17 years in ten EU countries, 3 August 2020 – 3 October 2021. Bundle et al. medRxiv (November 29, 2021).
Key findings:
- Pediatric cases and hospitalization rates increased as rates in adult age groups rose (Figure), but severe outcomes were rare.
- Among symptomatic children, 1.2% (n = 9,611) were hospitalized, 0.08% (n = 640) required intensive care, and 0.01% (n = 84) died.
- The risk of hospitalization was highest among children aged <1 year (13.1%) and lowest among those aged 8–13 years (0.6%).
- Most hospitalized children (83.7%) had no reported comorbidities; however, risks of hospitalization (adjusted odds ratio [aOR]29), intensive care (aOR 8.74), and death (aOR 26.85) were higher among children with any comorbidity compared with no comorbidities.
Methods: Analysis of pooled weekly surveillance data of COVID-19 symptomatic children aged 0-17 years (n = 820,404) reported by 10 European Union countries (Austria, Cyprus, Finland, Germany, Ireland, Italy, Luxembourg, Malta, Slovakia, and Sweden) from August 2020–October 2021. Hospitalization associated with comorbidities was estimated for 7 countries only. Limitations: Data on vaccination status of the children were not available; data restricted to symptomatic cases.
Implications: Risk of hospitalization and severe COVID-19 outcomes are higher among children with a potential comorbidity; CDC recommends COVID-19 vaccines for persons ages 5 years and older and emphasizes use of multiple COVID-19 prevention strategies to protect children <5 years of age.
Figure:
Note: Adapted from Bundle et al. Log weekly rates of reported (top) symptomatic COVID-19 cases and (bottom) hospital admissions per 100,000 population for individuals aged <1 year, 1–4 years, 5–11 years, 12–17 years, 18–49 years, 50–79 years, and ≥80 years, pooled from 10 EU countries, week 32, 2020–week 39, 2021. Licensed under CC-BY 4.0.
PREPRINTS (NOT PEER-REVIEWED)
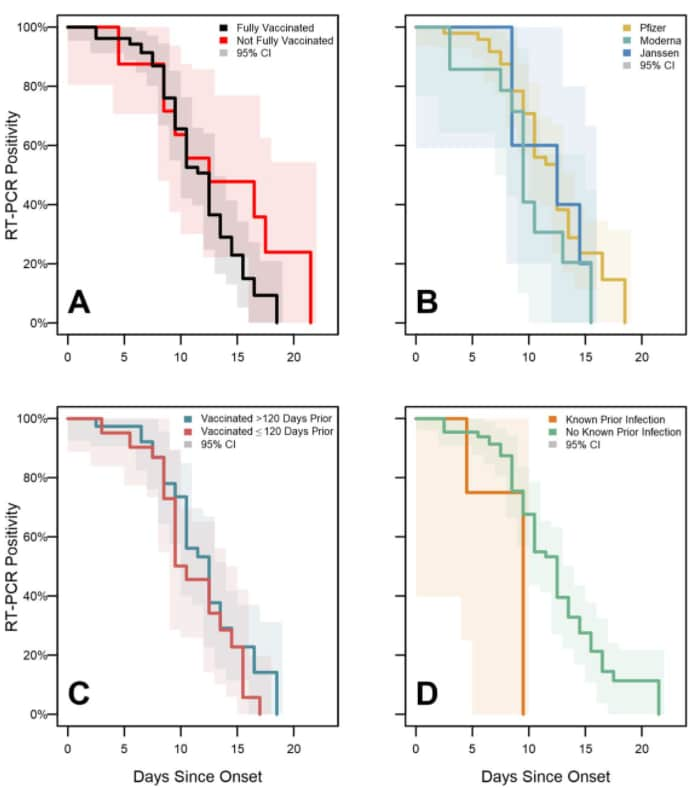
Transmission potential of vaccinated and unvaccinated persons infected with the SARS-CoV-2 Delta variant in a federal prison, July—August 2021. Salvatore et al. medRxiv (November 19, 2021).
Key findings:
- During a SARS-CoV-2 Delta (B.1.617.2) variant outbreak in a federal prison, duration of PCR positivity (median 13 days) and viral culture positivity (median 5 days) were similar for fully vaccinated and unvaccinated persons (Figure).
- Ct values in SARS-CoV-2 positive RT-PCR specimens increased with the number of days since onset, but no significant differences were observed among Ct values when stratified by vaccination status, vaccine product, time since vaccination, or history of prior SARS-CoV-2 infection.
Methods: Analysis of 978 nasal specimens serially collected from 95 incarcerated persons (78 received a primary series of an approved or authorized vaccine [“fully vaccinated”]) with confirmed SARS-CoV-2 infection July–August 2021. Duration of PCR positivity and viral culture positivity were assessed using survival analysis. Limitations: Small sample sizes underpower the statistical comparisons of vaccination status; potential misclassification of history of infection if not documented in Bureau of Prison medical system; results might not be generalizable.
Implications: Unvaccinated and vaccinated persons had similar duration of culturable virus suggesting similar transmission risks. In congregate settings like prisons, mitigation strategies, such as mask wearing and ventilation, should be applied when persons become infected with SARS-CoV-2 regardless of vaccination status.
Figure:
Note: Adapted from Salvatore et al. SARS-CoV-2 PCR test positivity survival curves for enrolled participants, federal prison, Texas, July 12–August 9, 2021, stratified by vaccination status (A), vaccine product (B), time since vaccination (C), and history of prior infection (D). Panels illustrate the results of Turnbull estimation survival functions with a primary endpoint of last positive PCR test result. Solid lines indicate nonparametric maximum likelihood estimates and shaded regions correspond to 95% CIs. U.S. Government work not subject to copyright.
Variants
- Probable transmission of SARS-CoV-2 Omicron variant in quarantine hotel, Hong Kong, China, November 2021. Gu et al. Emerging Infectious Diseases (December 3, 2021). The SARS-CoV-2 Omicron variant was detected in an asymptomatic, fully vaccinated traveler staying in a quarantine hotel in Hong Kong. Four days later, Omicron was detected in a 2nd fully vaccinated traveler, who had arrived from a different country on a different day and had stayed in a room across the corridor from the index patient. Viral genomes from these 2 cases differed only by 1 nucleotide. Retrospective investigation, including closed-circuit television camera footage, confirmed that neither case-patient left their room during the quarantine period.
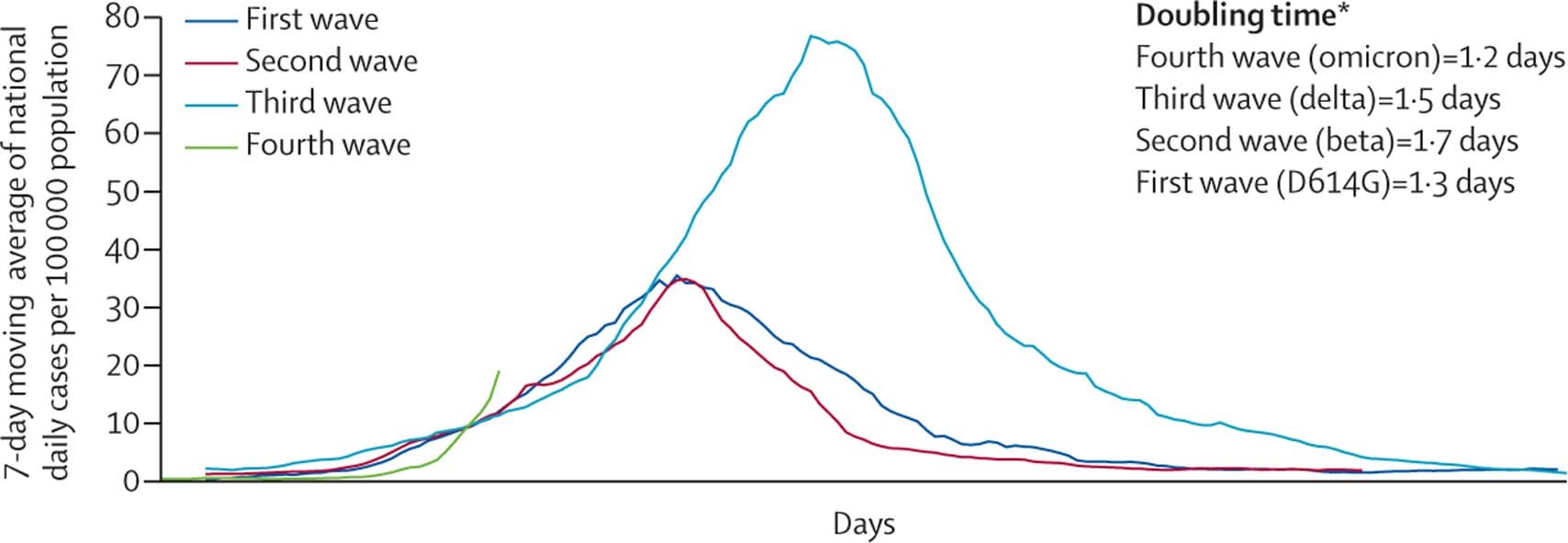
- Omicron SARS-CoV-2 variant: a new chapter in the COVID-19 pandemic. Karim et al. The Lancet (December 3, 2021). In a commentary that reported data from the Department of Health, Government of South Africa, COVID-19 cases in South Africa increased from a mean of 280 cases/day during the week before the detection of the Omicron variant to a mean of 800 cases/day the following week. In Gauteng province, data currently indicate that the number of cases doubled faster than was observed during the same phase of the previous 3 waves.
Note: Adapted from Karim et al. SARS-CoV-2 cases in first, second, third, and fourth waves, Gauteng Province of South Africa. *Doubling time for the first 3 days after the wave threshold of 10 cases per 100,000 population. 7-day moving average cases per 100,000 population up to December 1, 2021. Data are from the Department of Health, Government of South Africa. Permission request in process.
Vaccines
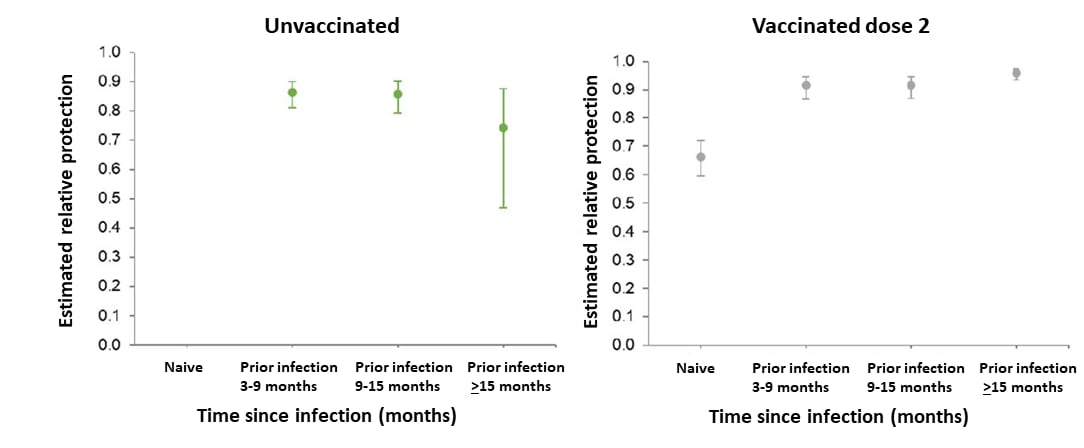
- Effectiveness and durability of protection against future SARS-CoV-2 infection conferred by COVID-19 vaccination and previous infection; findings from the UK SIREN prospective cohort study of healthcare workers March 2020 to September 2021. Hall et al. medRxiv (Preprint; December 1, 2021). Among 35,768 UK healthcare workers (December 2020–September 2021), adjusted vaccine effectiveness (aVE) among those who received 2 doses of BNT162b2 up to 6 weeks apart decreased from 85% (95% CI 71%-92%) 14–73 days following dose 2 to 58% (95% CI 40%-71%) >193 days after dose 2. Among those with prior infection, infection alone was associated with waning immunity over time. Prior infection plus 2 doses of BNT162b2 was associated with >90% protection from re-infection for at least 15 months after infection.
Note: Adapted from Hall et al. Protection following primary infection under different COVID-19 vaccination scenarios, up to 18 months following infection. Reference group is unvaccinated infection naïve. Licensed under CC-BY-NC-ND 4.0.
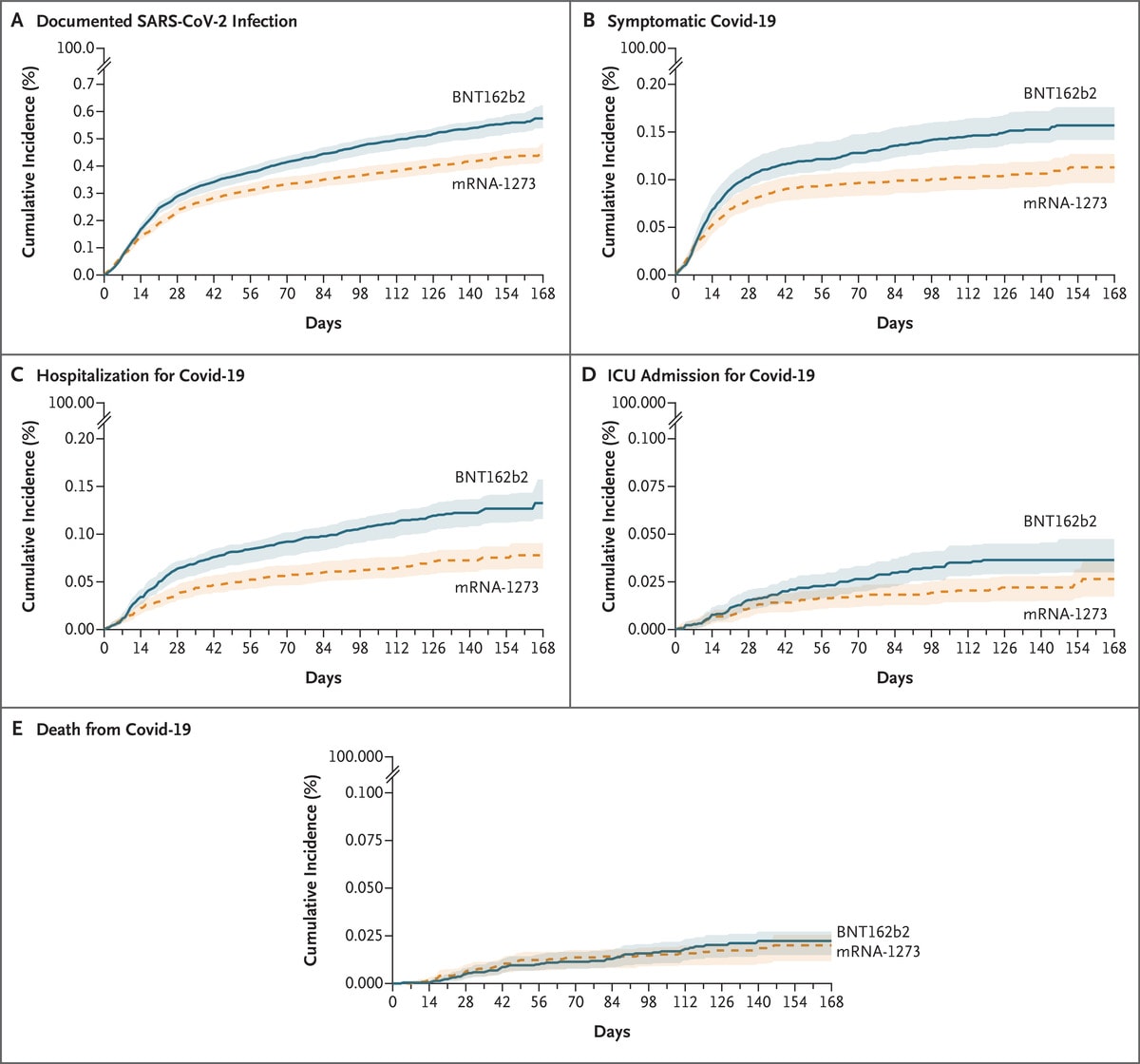
- Comparative effectiveness of BNT162b2 and mRNA-1273 vaccines in U.S. veterans.Dickerman et al. NEJM (December 1, 2021). In matched cohorts of BNT162b2 or mRNA-1273 vaccinated U.S. veterans (n = 219,842 per group) during January–July 2021, risk of COVID-19 hospitalization in the 24 weeks after vaccine dose 1 was low in both groups (1.33 events/1,000 persons [95% CI 1.16-1.57] and 0.78 events/1,000 persons [95% CI 0.64-0.91], respectively). However, compared with mRNA-1273, BNT162b2 vaccination was associated with a higher risk of hospitalization (RR 1.70, 95% CI 1.42-2.24) and ICU admission (RR 1.38, 95% CI 1.01-2.42).
Note: Adapted from Dickerman et al. Cumulative incidence of COVID-19 outcomes during a period marked by SARS-CoV-2 Alpha variant predominance (January 4–July 1, 2021). Shaded areas represent pointwise 95% confidence intervals. Permission request in process.
- Assessment of 4 doses of SARS-CoV-2 messenger RNA–based vaccine in recipients of a solid organ transplant. Kamar et al. JAMA Network Open (November 24, 2021). Among 37 solid organ transplant recipients in France, including 5 who had a weak response and 31 who had no response to the 1st 3 doses of BNT162b2 vaccine, a 4th dose was associated with continued suboptimal immune response. No adverse events were associated with the 4th
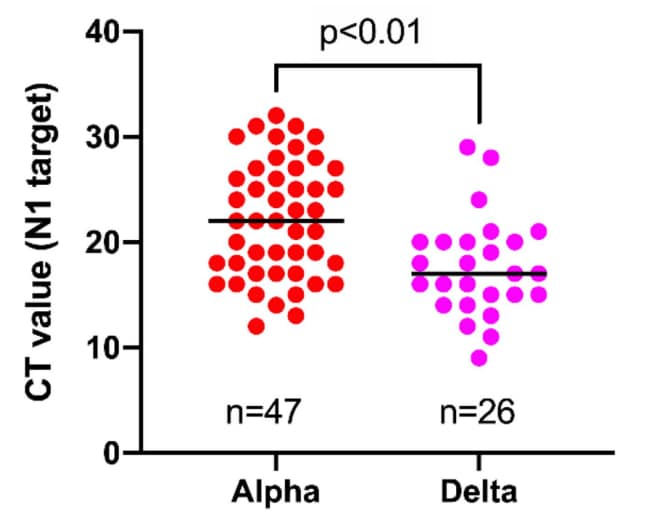
- Investigating SARS-CoV-2 breakthrough infections per variant and vaccine type. Dingemans et al. medRxiv (Preprint; November 24, 2021). Among fully vaccinated adults in the Netherlands during January–July 2021, SARS-CoV-2 breakthrough infections (n = 378) were most common among individuals vaccinated with ChAdOx1 (0.89%) followed by Ad26.COV2.S (0.46%) and mRNA vaccines (0.14%). Ct values were significantly lower (suggesting higher levels of viral RNA detected) (p<0.01) for breakthrough infections caused by the Delta variant (Ct value = 17) compared with Alpha variant (Ct value = 22). There was no association between SARS-CoV-2 genotype or vaccine type and disease severity.
Note: Adapted from Dingemans et al. Comparison of Ct values obtained by the same workflow for samples harboring Alpha versus Delta variant in breakthrough cases. Licensed under CC-BY-NC-ND 4.0.
Health Equity
- COVID-19 vaccination rates and attitudes among young adults with recent experiences of homelessness. Tucker et al. Journal of Adolescent Health (November 23, 2021). In a March–October 2021 survey of 134 young adults (ages 18–25 years; 68% male, 87% non-White) who recently experienced homelessness in Los Angeles County, 29% reported they were vaccinated. Among those not vaccinated, 57% strongly disagreed that they would get vaccinated if it was immediately available to them, and 45% strongly agreed that they would not trust the COVID-19 vaccine. Never being offered the vaccine was widely reported among unvaccinated participants (42%), suggesting insufficient outreach to young people experiencing homelessness.
From the Morbidity and Mortality Weekly Report (December 10, 2021).
- SARS-CoV-2 B.1.1.529 (Omicron) Variant — United States, December 1–8, 2021
- Booster and Additional Primary Dose COVID-19 Vaccinations Among Adults Aged ≥65 Years — United States, August 13, 2021–November 19, 2021
- Comparative Effectiveness and Antibody Responses to Moderna and Pfizer-BioNTech COVID-19 Vaccines among Hospitalized Veterans — Five Veterans Affairs Medical Centers, United States, February 1–September 30, 2021
- Community-Based Testing Sites for SARS-CoV-2 — United States, March 2020–November 2021
Disclaimer: The purpose of the CDC COVID-19 Science Update is to share public health articles with public health agencies and departments for informational and educational purposes. Materials listed in this Science Update are selected to provide awareness of relevant public health literature. A material’s inclusion and the material itself provided here in full or in part, does not necessarily represent the views of the U.S. Department of Health and Human Services or the CDC, nor does it necessarily imply endorsement of methods or findings. While much of the COVID-19 literature is open access or otherwise freely available, it is the responsibility of the third-party user to determine whether any intellectual property rights govern the use of materials in this Science Update prior to use or distribution. Findings are based on research available at the time of this publication and may be subject to change.