COVID-19 Science Update released: October 29, 2021 Edition 111

The COVID-19 Science Update summarizes new and emerging scientific data for public health professionals to meet the challenges of this fast-moving pandemic. Weekly, staff from the CDC COVID-19 Response and the CDC Library systematically review literature in the WHO COVID-19 databaseexternal icon, and select publications and preprints for public health priority topics in the CDC Science Agenda for COVID-19 and CDC COVID-19 Response Health Equity Strategy.
Section headings in the COVID-19 Science Update have been changed to align
with the CDC Science Agenda for COVID-19.
Here you can find all previous COVID-19 Science Updates.
PEER-REVIEWED
Vaccinating adolescents and children significantly reduces COVID-19 morbidity and mortality across all ages: a population-based modeling study using the UK as an example.external icon Shiri et al. Vaccines (October 15, 2021).
Key findings:
- In this modeling study, expanding vaccination eligibility in the United Kingdom from adults and vulnerable adolescents (base case scenario) to all adolescents (≥12 years) and children (5–11 years) reduced SARS-CoV-2 infections, hospitalizations, and deaths in the general population.
- Assuming an effective reproductive number (Reff) of 2.1 and equivalent vaccine coverage across age groups, expanding vaccination eligibility to all adolescents reduced infections by 6%–12% (depending on vaccine coverage) and deaths by 5%–12% compared with the base case scenario.
- Expanding vaccination eligibility to all adolescents and children reduced infections by 35%–60% and deaths by 37%–58%.
Methods: Estimates were based on an age-stratified, deterministic susceptible-exposed-infectious-removed (SEIR) compartmental model that assessed vaccination impact in the United Kingdom during July–December 2021 in the setting of a return to pre-pandemic social mixing levels. Sensitivity analyses included varying Reff and vaccination coverage. Limitations: Model estimates might not apply to United States; changes in inputs will change outcomes.
Implications: Vaccinating adolescents and children provides direct benefits to those groups and may also provide indirect benefit by reducing SARS-CoV-2 infections, morbidity, and mortality among other age groups.
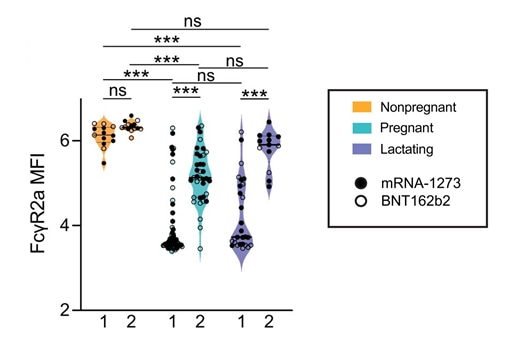
COVID-19 mRNA vaccines drive differential antibody Fc-functional profiles in pregnant, lactating, and non-pregnant womenexternal icon. Atyeo et al. Science Translational Medicine (October 19, 2021).
Key findings:
- Overall antibody titers were similar, but functionally distinct, among pregnant, lactating, and non-pregnant women following full vaccination with BNT162b2 (Comirnaty, Pfizer/BioNTech) and mRNA-1273 (Moderna).
- Following vaccine dose 1, pregnant and lactating persons had lower post-vaccination Fc receptor (FcR)-binding activity than non-pregnant women (Figure).
- Following dose 2, FcR activity was more comparable across groups, but statistically significant differences remained (Figure).
- High FcR-binding titers were detected in breastmilk after full vaccination.
Methods: Cohort study of 84 pregnant persons, 31 lactating persons, and 16 non-pregnant women aged 18–45 years, December 2020–February 2021. Serum specimens collected after 1st or 2nd doses of BNT162b2 or mRNA-1273 vaccines (and after both in a subset). SARS-CoV-2-specific anti-spike protein antibodies were assessed. Limitations: Small sample size; antibody responses in different gestational stages not examined; specimen collection timing after 2nd dose was different by vaccine; some patients contributed a specimen at single time point; immunologic indicators of protection from infection or disease are not yet established.
Implications: Pregnant and lactating persons may have modest immunological responses after the 1st dose of an mRNA vaccine but appear able to mount robust responses after the 2nd dose. Receipt of both primary series vaccine doses is critical for protecting the health of pregnant and lactating persons.
Figure
Note: Adapted from Atyeo et al. FcɣR2a-binding for non-pregnant (n = 13), pregnant (n = 64), and lactating (n = 28) persons following vaccine dose 1 and 2. The filled dots show the titer for persons who received mRNA-1273, and empty dots show the titer for persons who received BNT162b2. MFI = median fluorescence intensity. Data are presented as median ± IQR. * p <0.05, ** p <0.01, *** p <0.001. Licensed under CC BY 4.0.
Immunogenicity of standard and extended dosing intervals of BNT162b2 mRNA vaccineexternal icon. Payne et al. Cell (October 15, 2021).
Key findings:
- Among healthcare workers vaccinated with BNT162b2 (Comirnaty, Pfizer/BioNTech) and without prior SARS-CoV-2 infection, neutralizing antibody (NAb) titers against variants of concern were higher after a “long” (6–14 weeks) vs. “short” (2–5 weeks) interval between doses (Figure).
- At 4 weeks after the 2nd dose, NAb titers were 2–4-fold higher and memory B cells were 7-fold higher after a “long” interval series.
- NAb levels did not differ by dose interval in previously infected persons.
Methods: Prospective cohort study of healthcare workers who received BNT162b2, United Kingdom, December 9, 2020–May 23, 2021. A small subset was longitudinally assessed for antibody, B cell, and T cell responses. Limitations: Only BNT162b2 studied; small sample size; immunologic indicators of protection from infection or disease are not yet established.
Implications: Longer durations between 1st and 2nd doses of an mRNA primary series might enhance post-vaccination immune responses in those who have not been previously infected.
Figure:
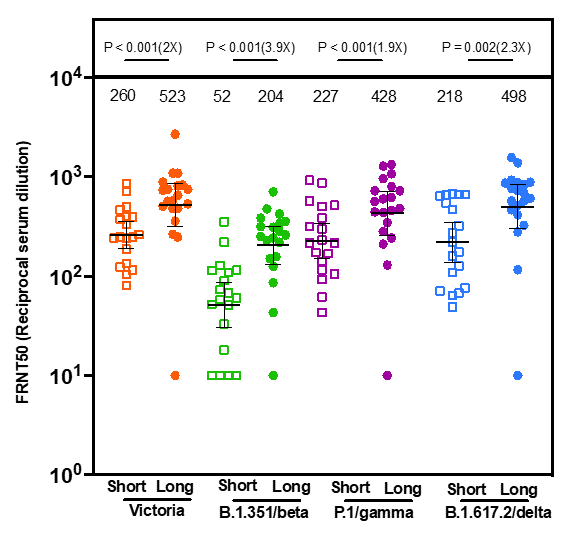
Note: Adapted from Payne et al. Comparison of neutralizing antibodies (y-axis) in participants who were not infected before vaccination against Victoria, B.1.351 (Beta), P.1 (Gamma), and B.1.617.2 (Delta) 4 weeks after 2nd dose in short (n = 19) and long (n = 20) interval participants. Geometric mean neutralizing titers are shown. Numbers in parentheses are fold changes. Numbers directly above each column are median neutralizing antibody titers. FRNT = Focus Reducing Neutralization Titer. Licensed under CC BY 4.0.
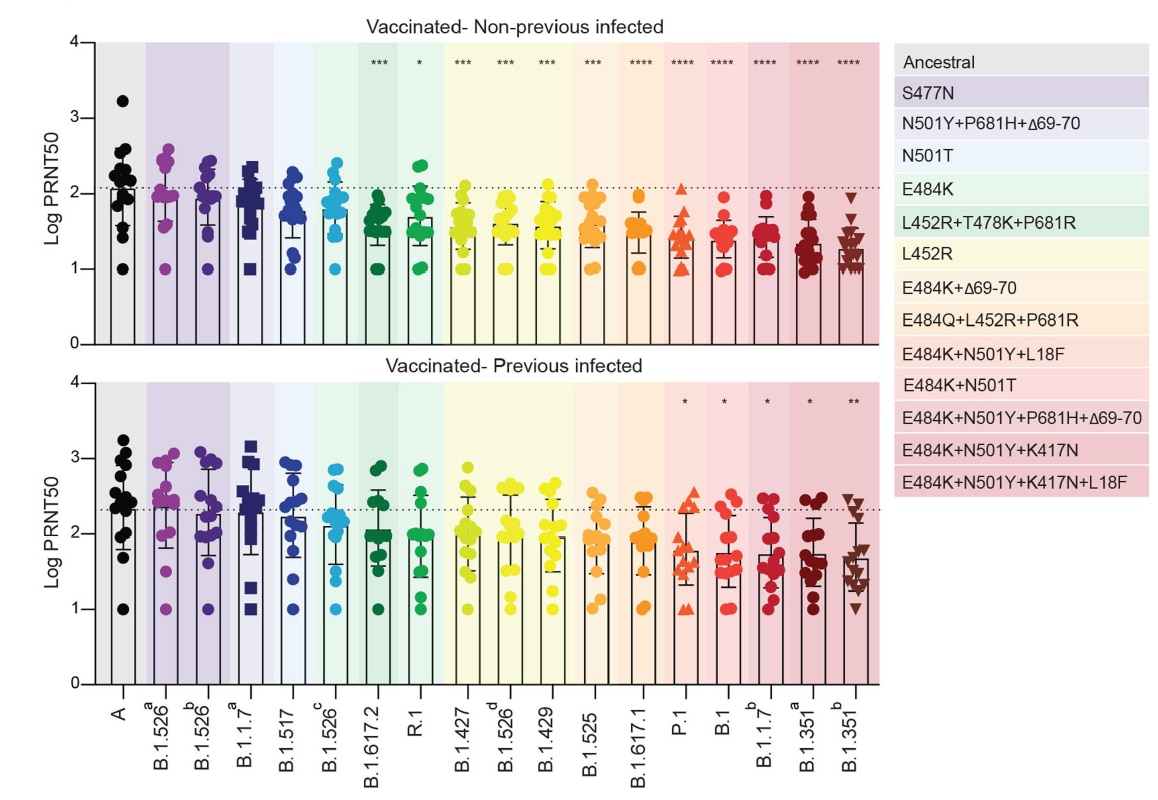
Impact of circulating SARS-CoV-2 variants on mRNA vaccine-induced immunity.external icon Lucas et al. Nature (October 11, 2021).
Key findings:
- In persons who received an mRNA vaccine, specific mutations in the SARS-CoV-2 spike gene were associated with reduced antibody neutralization capacity compared to the ancestral strain.
- Having both E484K and N501Y/T mutations (e.g., Beta [B.1.351] and Gamma [P.1] variants) decreased neutralization capacity 4.6–6.0-fold.
- Having the L452R mutation (e.g., Delta [B.1.617.2]) decreased neutralization capacity 2.5–4.1-fold.
- Overall neutralization capacity was higher in previously infected vaccinated individuals than in uninfected (naïve) vaccinated individuals, but both groups retained neutralization capacity against all variants (Figure).
- T cell activation markers measured by in vitro stimulation progressively increased after vaccination.
Methods: Serostudy of isolates from the Yale SARS-CoV-2 Genomic Surveillance program and clinical specimens from vaccinated healthcare workers (15 previously infected and 17 naïve), November 2020–January 2021. Immune responses to the SARS-CoV-2 ancestral strain, variants of concern/interest, and select lineages with key spike gene mutations were assessed. Limitations: Small sample size; immunologic indicators of protection from infection or disease are not yet established.
Implications: Post-vaccination neutralizing antibody responses to SARS-CoV-2 variants appear to be diminished but still provide neutralization capacity in fully vaccinated persons. COVID-19 vaccination remains critical.
Figure:
Note: Adapted from Lucas et al. Neutralizing activity to SARS-CoV-2 among vaccinated healthcare workers previously infected (bottom) or not previously infected/naïve (top). Plasma neutralization titers 28 days post-2nd vaccine dose locally circulating variants of concern or interest and other lineages compared to neutralization against ancestral lineage A virus (WA1, USA). Multiple isolates for some variants identified by a superscript letter. Bars represent mean values +/- standard deviations. Dotted line indicates the mean value of PRNT50 to the ancestral strain. Variants were grouped by specific spike gene mutation(s) and are colored according to the legend on the right. Each dot/marker represents a single individual. ****p <0.0001 ***p <0.001 **p <0.01*p <0.05. Permission request in process.
PEER-REVIEWED
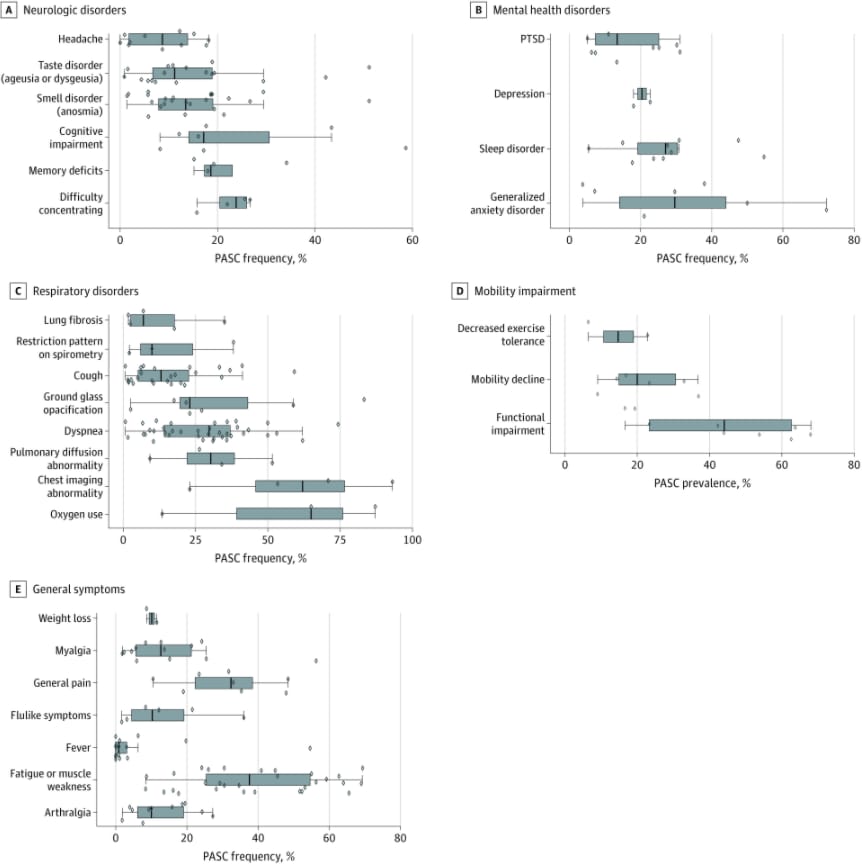
Short-term and long-term rates of postacute sequelae of SARS-CoV-2 infectionexternal icon. Groff et al. JAMA (October 13, 2021).
Key findings:
- Over half of COVID-19 survivors experienced at least 1 persistent post-acute sequela of COVID-19 (PASC) at 30 days–≥6 months after acute infection, based on data from a systematic review.
- The median PASC prevalence across studies was 54% at 1 month, 55% at 2–5 months, and 54% at ≥6 months.
- Among evaluated PASC symptoms, dyspnea (29.7%), cough (13.1%), and fatigue or muscle weakness (37.5%) were common (Figure).
- Difficulty concentrating, memory deficits, cognitive impairment, generalized anxiety, sleep disorders, and depression were also commonly reported (Figure).
Methods: Systematic review of 57 studies that included 250,351 COVID-19 survivors (79% were hospitalized for COVID-19) evaluated for 38 PASCs occurring 30 days–6 months after acute infection. Limitations: Meta-analysis not performed; may not be representative of patients with mild COVID-19 or patients from middle-to-low income countries; lack of standardized PASC definition; lack of standardized PASC reporting.
Implications: PASC (also called post-COVID conditions or “long COVID”) appear to be common following COVID-19 infection, may significantly affect survivors’ health, and may place an additional burden on healthcare systems.
Figure:
Note: Adapted from Groff et al. Neurologic (A), mental health (B), respiratory (C), mobility (D), and general (E) post-acute sequelae of COVID-19 (PASC). The vertical line in each box plot is the median value for the outcome of interest. The width of the box is the IQR. The whiskers extend to the smallest and largest observations within 1.5 times the IQR of the quartiles. The diamonds represent point estimates for each study included. Licensed under CC BY.
Assessment of cognitive function in patients after COVID-19 infectionexternal icon. Becker et al. JAMA Network Open (October 22, 2021).
Key findings:
- Among 740 adult patients with prior COVID-19 infection, ~24% had memory impairment (encoding and recall), 18% had impaired processing speed, and 16% had impaired executive functioning (16%).
- Adults who were hospitalized for COVID-19 were more likely to have impairments in attention (aOR = 2.8, 95% CI 1.3-5.9), memory encoding (aOR = 2.3, 95% CI 1.3-4.1), memory recall (aOR = 2.2, 95% CI 1.3-3.8), and executive functioning (aOR = 1.8, 95% CI 1.0-3.4) compared with those treated in outpatient settings.
Methods: Cross-sectional study of post-COVID-19 cognitive functioning (mean 7.6 months [SD 2.7] after acute infection) among adults (mean age = 49 years) with no history of dementia. Validated neuropsychological measures used; impairment defined as a z-score of ≤ 1.5 SD below the age-, educational level-, and sex-adjusted norms. Limitations: Sample may not be representative; lack of non-COVID-19 comparison group limits ability to attribute causality to COVID-19 (as opposed to potential effects from severe illness or hospitalization).
Implications: The substantial proportion of patients with cognitive impairment several months after COVID-19 highlights the importance of continued efforts to prevent SARS-CoV-2 infection and reduce severe disease.
PEER-REVIEWED
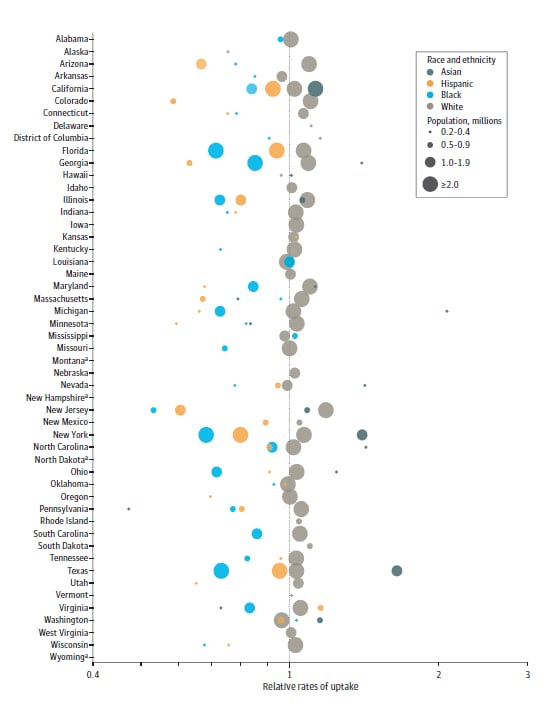
Quantifying and benchmarking disparities in COVID-19 vaccination rates by race and ethnicityexternal icon. Reitsma et al. JAMA Network Open (October 20, 2021).
Key findings:
- Among most states, relative vaccine uptake among White adults as of March 31, 2021 was a median of 1.3 times that of Black or African American (IQR 1.2-1.4) and Hispanic or Latino adults (1.1-1.6) (Figure).
- Modeling suggested that equalizing uptake and prioritizing disadvantaged census tracts would have eliminated vaccination disparities between White and Hispanic/Latino adults by July 2021.
- Vaccination disparities between White and Black or African American adults would have been reduced by 76% by July 2021.
Methods: Cross-sectional estimation of relative vaccine uptake, defined as observed share of vaccinations for a racial or ethnic group divided by the expected share of vaccinations (at least 1 dose and proportional to group size), as of March 31, 2021. Vaccination scale-up modeled by census tract under 3 scenarios: (1) continuation of observed disparities; (2) equal uptake by racial and ethnic groups (equalized uptake); and (3) equalized uptake + geographic prioritization. Limitations: Vaccine uptake measured during early phase of vaccine roll-out; incomplete race and ethnicity reporting in state vaccination data; racial and ethnic groups with fewer than 200,000 people in a state not included.
Implications: Modeling data suggest that reducing disparities in vaccine uptake might be achieved by geographic prioritization and group tailoring of vaccine promotion efforts.
Figure:
Note: Adapted from Reitsma et al. Relative rates of COVID-19 vaccination uptake as of March 31, 2021 by race and ethnicity (Asian, Hispanic, Black, White) and state on a log scale. a = state vaccination data not reported by race and ethnicity as of March 31, 2021. Licensed under CC BY.
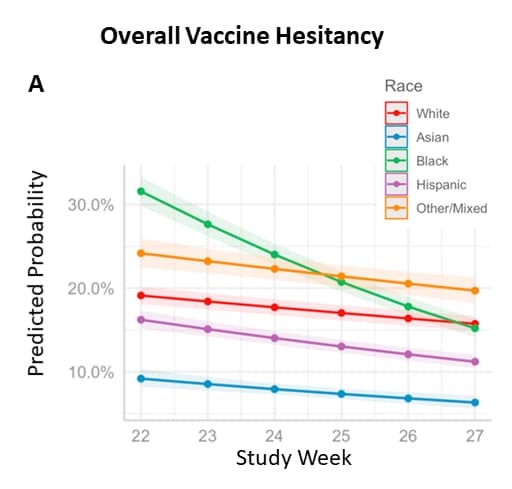
Hesitancy in the time of coronavirus: Temporal, spatial, and sociodemographic variations in COVID-19 vaccine hesitancyexternal icon. Liu et al. Social Science & Medicine (September, 2021)
Key findings:
- In a large nationally representative household survey, overall vaccine hesitancy was higher among Black or African American persons and lower among Hispanic/Latino and Asian persons compared with White persons.
- Vaccine hesitancy decreased most sharply among Black or African American persons (Figure 1).
- Black or African American women reported more vaccine hesitancy than Black men (Figure 2).
- Dimensions of hesitancy (confidence, circumspection, and complacency) differed by race, ethnicity, gender, and over time.
Methods: Descriptive and multilevel modeling of 6 rounds (weeks 22 to 27) of the U.S. Household Pulse survey (n = 443,680) conducted during January to March 2021. Limitations: Data collected when many respondents were not eligible for vaccination.
Implications: Vaccination promotion strategies may need to be tailored to the concerns of specific populations to improve vaccine uptake.
Figure:
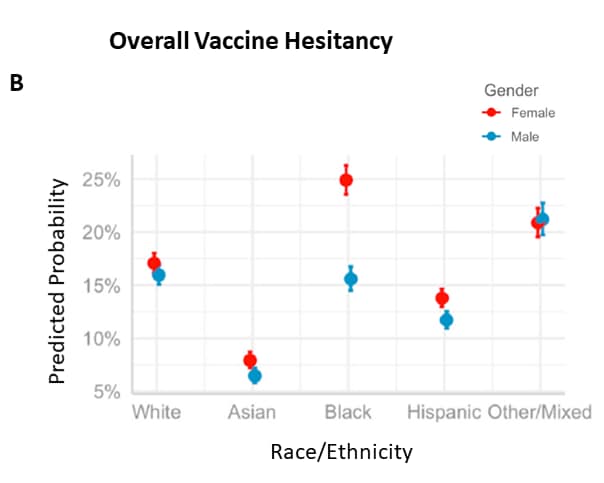
Hesitancy in the time of coronavirus: Temporal, spatial, and sociodemographic variations in COVID-19 vaccine hesitancyexternal icon. Liu et al. Social Science & Medicine (September, 2021)
Key findings:
- In a large nationally representative household survey, overall vaccine hesitancy was higher among Black or African American persons and lower among Hispanic/Latino and Asian persons compared with White persons.
- Vaccine hesitancy decreased most sharply among Black or African American persons (Figure 1).
- Black or African American women reported more vaccine hesitancy than Black men (Figure 2).
- Dimensions of hesitancy (confidence, circumspection, and complacency) differed by race, ethnicity, gender, and over time.
Methods: Descriptive and multilevel modeling of 6 rounds (weeks 22 to 27) of the U.S. Household Pulse survey (n = 443,680) conducted during January to March 2021. Limitations: Data collected when many respondents were not eligible for vaccination.
Implications: Vaccination promotion strategies may need to be tailored to the concerns of specific populations to improve vaccine uptake.
Figure:
Note: Adapted from Liu et al. A) Predicted probability of vaccine hesitancy by survey week and race/ethnicity (for White, Asian, Black or African American, Hispanic or Latino persons, and persons reporting Other/Multiple race/ethnicities), Household Pulse Survey week 22 (January 6–18, 2021) through week 27 (March 17–29, 2021). B) Predicted probability of vaccine hesitancy by race and gender (female, male). Licensed under CC-BY-NC-ND 4.0.
Vaccines
- Effectiveness of the mRNA-1273 vaccine during a SARS-CoV-2 Delta outbreak in a prisonexternal icon. Chin et al. NEJM (October 20, 2021). Among 827 prison residents (468 fully vaccinated with mRNA-1273, 359 unvaccinated), the vaccine effectiveness (VE) during a Delta variant-driven outbreak (July–August 2021) was 56.6% (95% CI 42.0%-67.5%) against any infection and 84.2% (95% CI 56.4%-94.3%) against symptomatic infection. Among residents who had a previous confirmed infection, VE against subsequent infection was 80.5% (95% CI 52.8%-92.0%).
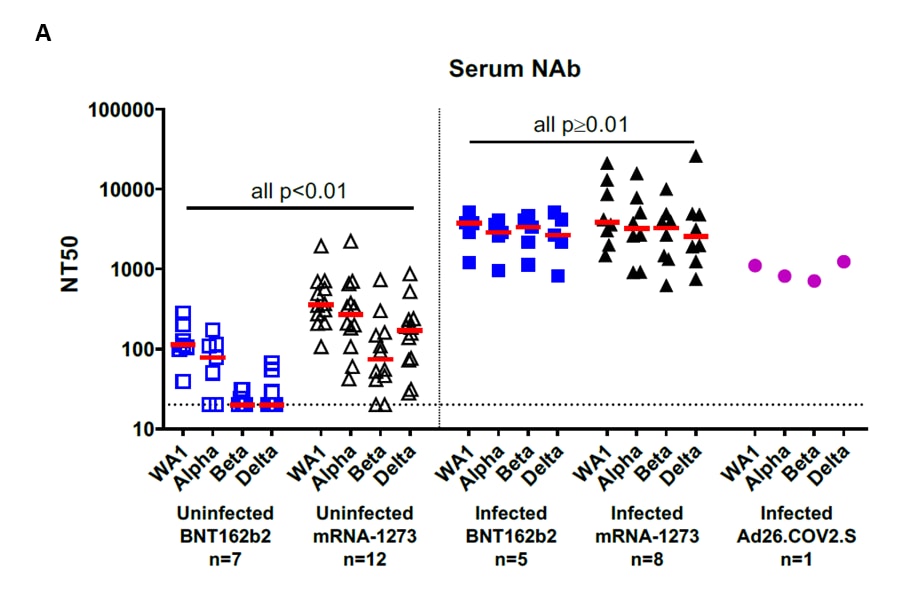
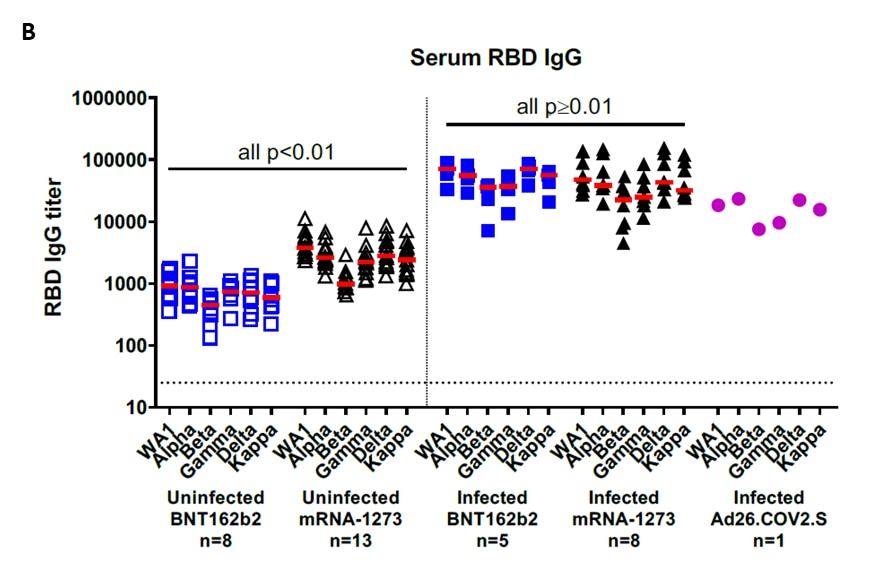
- Immune responses in fully vaccinated individuals following breakthrough infection with the SARS-CoV-2 Delta variant in Provincetown, Massachusettsexternal icon. Collier et al. medRxiv (Preprint; October 21, 2021). Published in Science Translational Medicine as Characterization of immune responses in fully vaccinated individuals after breakthrough infection with the SARS-CoV-2 Delta variantexternal icon (March 8, 2022). In 35 fully vaccinated adults, individuals with breakthrough infections (n = 14) had 20- to 30- fold higher receptor binding domain (RBD)-specific IgG titers against SARS-CoV-2 WA1/2020, Alpha (B.1.1.7), Beta (B.1.351), Gamma (P.1), Delta (B.1.617.2), and Kappa (B.1.617.1) compared to those uninfected (n = 21). Neutralizing antibodies against variants were 13- to 73- fold higher.
Note: Adapted from Collier et al. Serum antibody responses in BNT162b2, mRNA-1273, and Ad26.COV2.S vaccinated individuals as part of the SARS-CoV-2 outbreak investigation in Provincetown, Massachusetts. Vaccinated uninfected (open symbols) and vaccinated infected (filled symbols) individuals are shown. A) Pseudovirus neutralization titers measured as 50% reduction (NT50) of luciferase expression. B) IgG titers to SARS-COV-2 RBD variants. Medians (bar) for each variant are displayed. Dotted line indicates limit of detection. P values compare participants who received BNT162b2 to those who received mRNA-1273. Licensed under CC-BY-ND 4.0.
- COVID-19 vaccination during pregnancy and first-trimester miscarriageexternal icon. Magnus et al. NEJM (October 19, 2021). COVID-19 vaccination was not associated with increased odds of 1st trimester miscarriage in a case-control study using registry data (February–August 2021) of Norwegian women who miscarried (cases; n = 4,521) or with ongoing pregnancy (controls; n = 13,956). Results were similar for sub-analyses by number of doses received or vaccine type (BNT162b2, mRNA-1273, or ChAdOx1), and when limited to healthcare personnel only or women with at least 8 weeks of follow-up after confirmed pregnancy.
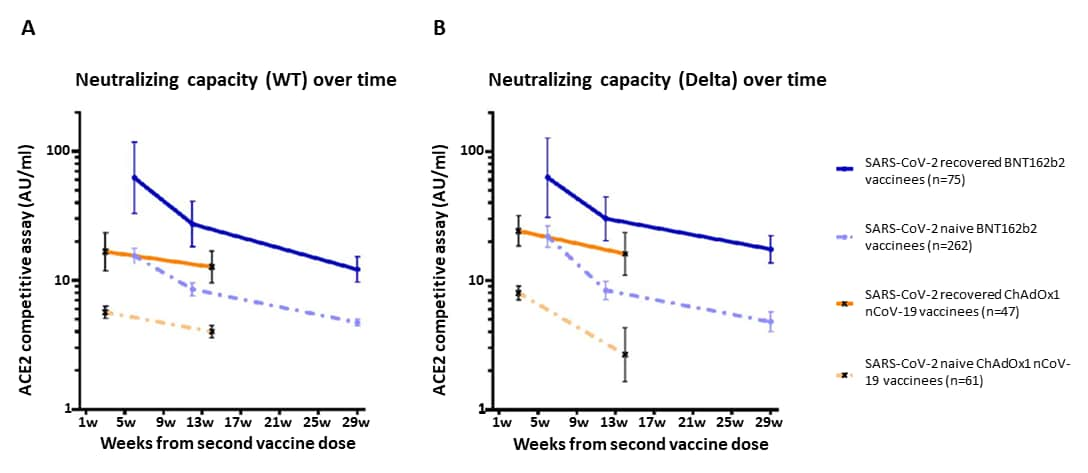
- Impact of SARS-CoV-2 infection on longitudinal vaccine immune responsesexternal icon. Havervall et al. medRxiv (Preprint; October 19, 2021). Published in Clinical & Translational Immunology as Impact of SARS-CoV-2 infection on vaccine-induced immune responses over timeexternal icon (April 18, 2022). Among 517 healthcare workers in Sweden (April 2020–July 2021), vaccination (BNT162b2 or ChAdOx1) of persons with prior SARS-CoV-2 infection resulted in higher neutralizing antibodies for at least 3–7 months compared with SARS-CoV-2 naïve vaccinees (all timepoints p<0.001). Neutralizing antibodies against variants of concern (e.g., Alpha [B.1.1.7], Delta [B.1.617.2]) were 2- to 3-fold higher in vaccinees with prior SARS-COV-2 infection compared to naïve vaccinees.
Note: Adapted from Havervall et al. Pseudo-neutralizing antibody titers over 7 months following the 2nd BNT162b2 dose and 3 months following the 2nd ChAdOx1 dose in SARS-CoV-2 recovered and naïve vaccinees against B) wild-type and C) Delta variant SARS-CoV-2. Dots and crosses represent geometric mean titers and bars represent 95 % CI. Solid lines represent SARS-CoV-2 recovered vaccinees and dotted lines represent SARS-CoV-2 naïve vaccinees. WT = wild type; AU = arbitrary units. Used by permission of authors.
- Effectiveness of BNT162b2 vaccine against Delta variant in adolescentsexternal icon. Reis et al. NEJM (October 20, 2021). Among 94,354 vaccinated Israeli adolescents 12–18 years and matched controls, vaccine effectiveness within 21 days after BNT162b2 dose 2 was 90% (95% CI 88%-92%) against SARS-CoV-2 infection and 93% (95% CI 88%-97%) against symptomatic illness when the Delta (B.1.617.2) variant predominated (June–October 2021).
Variants
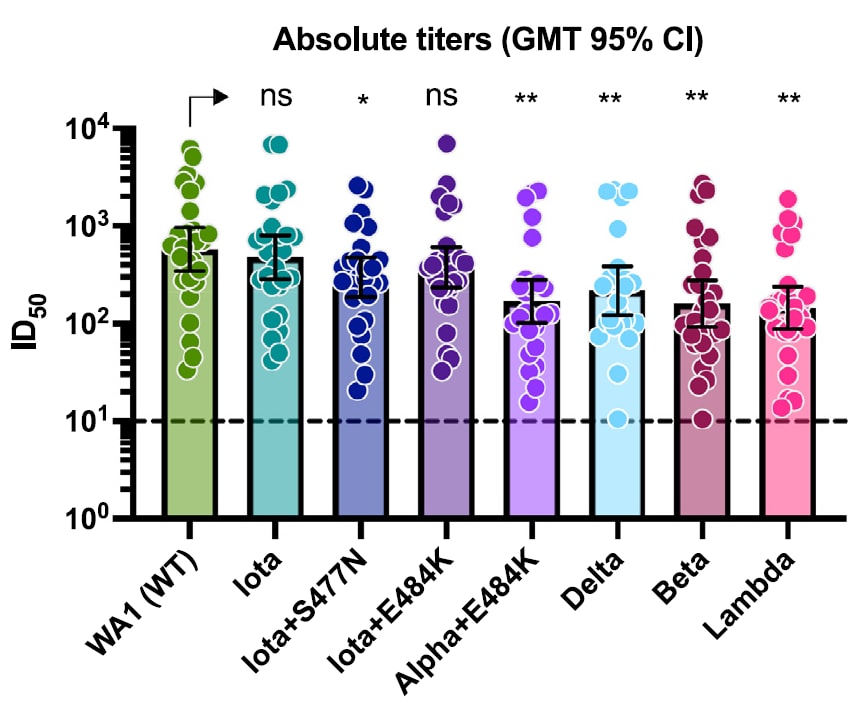
- Evidence for retained spike-binding and neutralizing activity against emerging SARS-CoV-2 variants in serum of COVID-19 mRNA vaccine recipientsexternal icon. Carreño et al. EBioMedicine (October 20, 2021). In serum from 30 vaccinated individuals (15 mRNA-1273, 15 BNT162b2) tested against SARS-CoV-2 variants of concern/interest, small decreases were found in neutralization of Iota (B.1.526)+S477N (1.9-fold; p <0.5) and Delta (B.1.617.2) (2.6-fold, p <0.01) compared with neutralization of wild type WA1 isolate. Greater reductions were found for a Lambda (C.37) subvariant (4.0-fold, p <0.01), Beta (B.1.351) (3.6-fold, p <0.01) and Alpha (B.1.1.7)+E484K (3.4-fold, p <0.01).
Note: Adapted from Carreño et al. Neutralizing activity of post-mRNA vaccination sera against different variants of concern/interest. Geometric mean titers (GMT) with the 95% confidence intervals are depicted for each viral variant. ns = not significant, * p <0.05; ** p <0.01. Licensed under CC-BY-NC-ND.
Natural History, Reinfection, and Health Impact
- Protective immunity after natural infection with Severe Acute Respiratory Syndrome Coronavirus-2 (SARS-CoV-2) – Kentucky, USA, 2020external icon. Spicer et al. International Journal of Infectious Diseases (October 12, 2021). Among 41,647 state residents with documented SARS-CoV-2 infection during March–August 2020, the protective effect against reinfection 3–9 months after initial infection was 75%–86% for those aged 20–59 years and 53%–81% for those ≥60 years. Factors associated with repeat positive testing included work or residence in long-term care (aRR 4.35, 95% CI 3.45-5.26) and absence of symptoms during the initial infection (aRR 2.31, 95% CI 1.93-2.75).
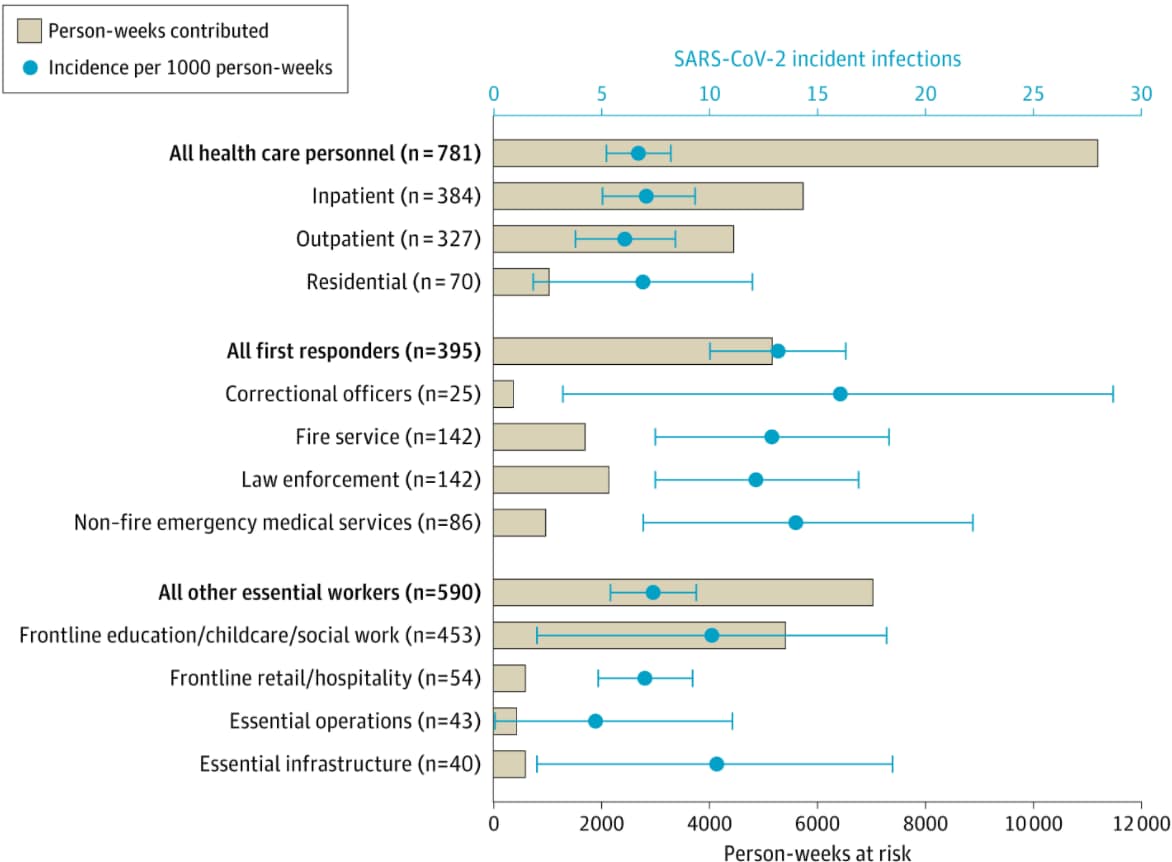
- Incidence of SARS-CoV-2 infection among health care personnel, first responders, and other essential workers during a prevaccination COVID-19 surge in Arizonaexternal icon. Ellingson et al. JAMA (October 22, 2021). In a prospective cohort of 1,766 unvaccinated seronegative workers tested weekly during July 2020–March 2021, first responders had a significantly higher incidence of SARS-CoV-2 infection than healthcare personnel (IRR 2.01, 95% CI 1.44-2.79), even after controlling for sociodemographic characteristics and underlying health and exposure indicators (aIRR 1.60, 95% CI 1.07-2.38). Incidence was highest among correctional officers and non-fire emergency medical service workers.
Note: Adapted from Ellingson et al. Person-weeks contributed and crude incidence of SARS-CoV-2 infection per 1000 person-weeks at risk by occupation. Essential operations includes workers in sectors requiring in-person work (e.g., post office or 911 call centers) and essential infrastructure includes workers in utility and municipal services (e.g., waste management, electricity, water). Licensed under CC BY.
Health Equity
- High-risk outreach for COVID-19 mortality reduction in an indigenous communityexternal icon. Stone et al. American Journal of Public Health (October 14, 2021). In response to a large outbreak in a rural Native American community, officials augmented contact tracing, prioritized the highest-risk contacts for tracing, added immediate and home-based contact testing, and partnered with the tribal government to integrate public health outreach and direct medical care. These interventions contributed to a low community case fatality rate (CFR) (1.3% as of February 15, 2021), compared with all Native American persons in the state (3.2%) and the overall CFR for the state (1.9%).
From the Morbidity and Mortality Weekly Report (October 29, 2021)
- Laboratory-Confirmed COVID-19 Among Adults Hospitalized with COVID-19–Like Illness with Infection-Induced or mRNA Vaccine-Induced SARS-CoV-2 Immunity — Nine States, January–September 2021
- The Advisory Committee on Immunization Practices’ Interim Recommendations for Additional Primary and Booster Doses of COVID-19 Vaccines — United States, 2021
- Severity of Disease Among Adults Hospitalized with Laboratory-Confirmed COVID-19 Before and During the Period of SARS-CoV-2 B.1.617.2 (Delta) Predominance — COVID-NET, 14 States, January–August 2021
- COVID-19 Vaccination and Non–COVID-19 Mortality Risk — Seven Integrated Health Care Organizations, United States, December 14, 2020–July 31, 2021
Disclaimer: The purpose of the CDC COVID-19 Science Update is to share public health articles with public health agencies and departments for informational and educational purposes. Materials listed in this Science Update are selected to provide awareness of relevant public health literature. A material’s inclusion and the material itself provided here in full or in part, does not necessarily represent the views of the U.S. Department of Health and Human Services or the CDC, nor does it necessarily imply endorsement of methods or findings. While much of the COVID-19 literature is open access or otherwise freely available, it is the responsibility of the third-party user to determine whether any intellectual property rights govern the use of materials in this Science Update prior to use or distribution. Findings are based on research available at the time of this publication and may be subject to change.