COVID-19 Science Update released: October 13, 2020 Edition 56

The COVID-19 Science Update summarizes new and emerging scientific data for public health professionals to meet the challenges of this fast-moving pandemic. Weekly, staff from the CDC COVID-19 Response and the CDC Library systematically review literature in the WHO COVID-19 databaseexternal icon, and select publications and preprints for public health priority topics in the CDC Science Agenda for COVID-19 and CDC COVID-19 Response Health Equity Strategy.
Here you can find all previous COVID-19 Science Updates.
PEER-REVIEWED
Frequent neurologic manifestations and encephalopathy‐associated morbidity in COVID‐19 patientsexternal icon. Liotta et al. Annals of Clinical and Translational Neurology (October 5, 2020).
Key findings:
- 82.3% of hospitalized COVID-19 patients had neurologic manifestations during their disease course.
- The most frequent neurologic manifestations were myalgia (44.8%), headache (37.7%), and encephalopathy (31.8%).
- Patients with severe COVID‐19 were more likely to have neurologic manifestations, (adjusted OR [aOR] 4.02, 95% CI 2.04-8.89, p <0.001) including encephalopathy (aOR 131, 95% CI 61.2-310, p <0.001).
- Patients with encephalopathy had longer median hospital stays, 17 (IQR 11–25) vs 5 (IQR 3–8) days (p <0.001), and higher mortality within 30 days of hospitalization, 21.7% vs 3.2% (p <0.001).
Methods: Retrospective chart review for neurologic manifestations in 509 consecutive patients with RT-PCR-confirmed SARS-CoV-2 infection admitted to a Chicago hospital system between March 5 and April 6, 2020. COVID-19 neurologic manifestations were compared across severity strata and associated with mortality, adjusted by demographic and clinical variables. Limitations: Some neurologic manifestations might not have been identified or recorded; <6% patients evaluated by neurologists, neurosurgeons, or by neurologic imaging (brain CT in 9.0% and MRI in 3.1% of patients).
Implications: Neurologic manifestations may be frequent in hospitalized COVID-19 patients and may be associated with disease severity; encephalopathy may contribute to increase morbidity and mortality. Extent of long-term burden from COVID-19-related encephalopathy remains to be determined.
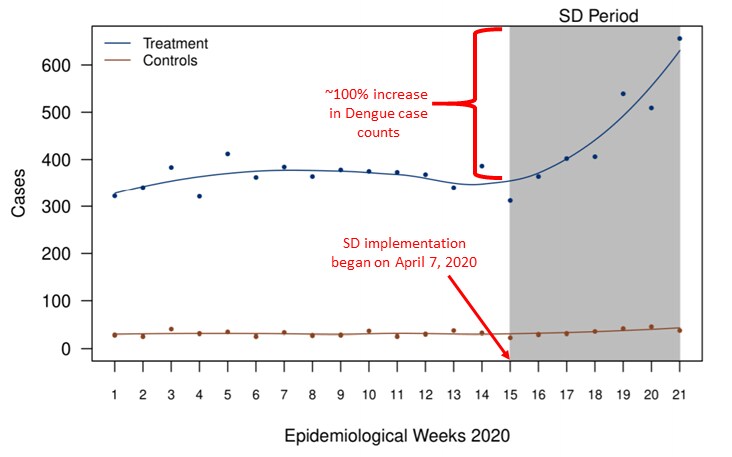
Increased dengue transmissions in Singapore attributable to SARS-CoV-2 social distancing measures.external icon Lim et al. Journal of Infectious Diseases (September 30, 2020).
Key findings:
- Dengue case counts increased approximately 100% among persons aged 5–65 years, after implementation of strictly enforced social distancing (SD) (Figure).
- 37.2% (95% CI 19.9%-49.8%), of the increase in dengue cases was attributable to implementation of SD.
Methods: Weekly dengue case counts (2003 through June 1, 2020) were compared before and after implementation of SD policies in Singapore that began April 7, 2020. Case counts were compared between persons aged 5–65 years (treatment group, persons least likely to be home during the day before SD) and persons <5 and >65 years (control group, more likely to be home during the day). The analysis controlled for time trends, seasonality, year fixed effects, temperature and humidity. Limitations: Cross-immunity between dengue serotypes was not accounted for as a potential confounder.
Implications: Because mosquito breeding grounds are concentrated in residential areas in Singapore, increased time spent at home during the implementation of COVID-19 SD potentially resulted in increased exposure to mosquito vectors, and, consequently to increased dengue risk. Measures to mitigate risk of dengue transmission should be considered when implementing SD in dengue-endemic countries.
Figure:
Note: Adapted from Lim et al. Dengue case counts between January 1 and May 23, 2020 (epidemiological weeks 1–21) for treatment and control groups. Treatment, persons aged 5–65 years; Controls, persons aged ≤5 and ≥70 years. Licensed under CC-BY-NC-ND.
PEER-REVIEWED
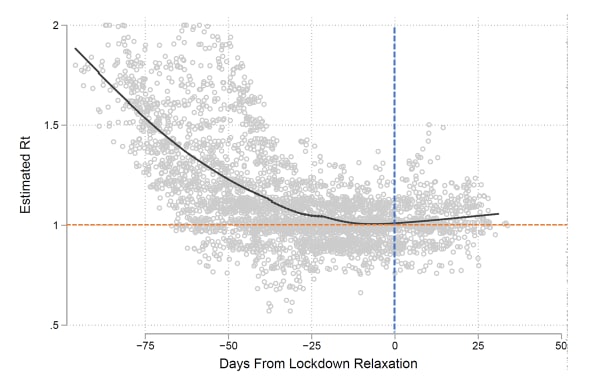
COVID-19 transmission in the U.S. before vs. after relaxation of statewide social distancing measuresexternal icon. Tsai et al. Clinical Infectious Diseases (October 3, 2020).
Key findings:
- Two months prior to relaxing social distancing (SD) policies, most states saw a decline in cases with the mean effective reproduction number (Rt, the average number of new infections caused by a single infected person) declining by 0.012, per day (Figure).
- In all but nine states, the reversal of SD measures resulted in an increase in cases within eight weeks with an estimated Rt >1.0.
- Increases in Rt were apparent regardless of the kind of SD rescinded (easing of work restrictions, rescission of statewide restriction on internal movement) or the epidemic severity.
Methods: Analysis of state-level data on implementation and relaxation of SD measures between March 10 and July 15, 2020 in the US. The effect that relaxing SD had on epidemic control was determined using linear regression and Rt was estimated. Limitations: Potential for confounding by factors that occurred simultaneously with relaxation of SD measures which could also influence the Rt.
Implications: These findings could inform policymakers in their evaluation of when and how to reverse SD measures.
Figure:
Note: Tsai et al. Estimated Rt for each state before and after relaxation of SD measures. Gray dots indicate estimated Rt for each state over time; Black line is a smoothed regression line; Blue dotted line indicates the day SD was relaxed; Orange dotted line indicates Rt = 1 (cases are endemic); Rt >1 indicates increasing cases and Rt <1 indicates decreasing cases. Reproduced by permission of Oxford University Press on behalf of the Infectious Diseases Society of America. Please visit: https://doi.org/10.1093/cid/ciaa1502external icon.
PEER-REVIEWED
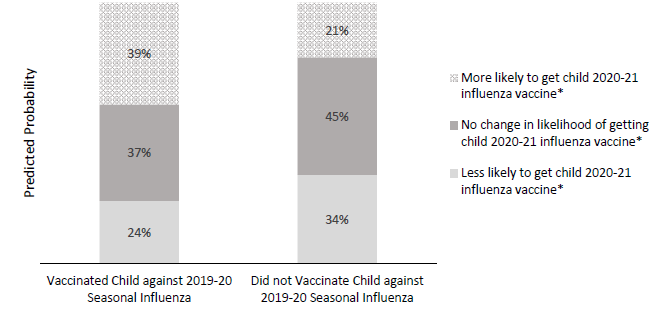
COVID-19 and parent intention to vaccinate their children against influenza.external icon Sokol et al. Pediatrics (October 1, 2020).
Key findings:
- Parental intention to vaccinate children against influenza significantly differed between parents whose children received the 2019–2020 influenza vaccine compared with those whose children did not (p <0.001).
- Among parents whose children did not receive the 2019–2020 influenza vaccine, 34% (95% CI 30%-37%) reported that the COVID-19 pandemic made them less likely to vaccinate their child against influenza during the 2020–2021 influenza season (Figure).
Methods: In May 2020, a convenience sample of 2,164 parents of children 6 months to 5 years of age completed an online survey comparing their children’s 2019-2020 influenza vaccination status with parental intention to vaccinate their children against influenza during the 2020–2021 influenza season. A multivariate model was adjusted for child and caregiver demographics. Limitations: Convenience sample may not be generalizable; survey limited to people with internet access.
Implications: The COVID-19 pandemic does not seem to uniformly motivate parents to vaccinate their children against influenza. Outreach and education to parents may help them better understand the importance of influenza vaccination.
Figure:
Note: Adapted from Sokol et al. Predicted probabilities for parents’ intentions for children’s influenza vaccination as a result of the COVID-19 pandemic, according to 2019–2020 influenza vaccination status. *Indicates significant differences in predicted probabilities between parents whose child did vs did not receive the 2019–2020 influenza vaccination. Reproduced with permission from Pediatrics, Vol. 146, Page e2020022871, Copyright © 2020 by the AAP.
Lopinavir–ritonavir in patients admitted to hospital with COVID-19 (RECOVERY): A randomised, controlled, open-label, platform trialexternal icon. The RECOVERY Collaborative Group. Lancet (October 5, 2020).
Key findings:
- No significant difference was found between hospitalized patients with COVID-19 assigned to lopinavir–ritonavir and patients assigned to usual care with regard to:
- Death within 28 days (374 [23%] vs 767 [22%]) (Figure).
- Time to discharge alive from hospital (median 11 days [IQR 5 to >28] in both groups).
- Proportion discharged alive within 28 days (RR 0.98, 95% CI 0.91-1.05, p = 0.53).
- Results were consistent across all prespecified subgroups of patients stratified by age, sex, ethnicity, duration of symptoms, respiratory support at randomization, and baseline predicted risk of death.
Methods: Multi-center, open-label, randomized, controlled trial of hospitalized COVID-19 patients assigned to lopinavir-ritonavir (n = 1,616) or to regular care (n = 3,424) as part of the RECOVERY trialexternal icon. The primary outcome was 28-day all-cause mortality. Limitations: Relatively few intubated patients enrolled.
Implications: Consistent with results from previously publishedexternal icon smaller trial and interim results from the WHO’s SOLIDARITY trialexternal icon, these findings do not support lopinavir–ritonavir treatment for patients hospitalized with COVID-19. An accompanying editorialexternal icon argues that antivirals alone might be insufficient for severely ill patients and that combination therapy with antivirals and immunomodulators should be evaluated for severe COVID-19.
Figure:
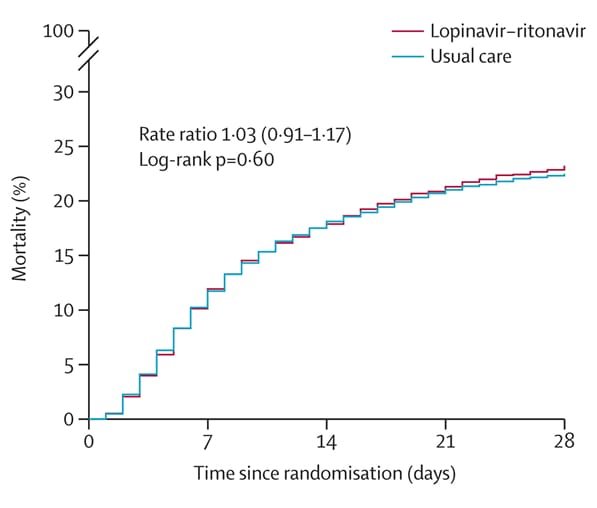
Note: Adapted from The RECOVERY Collaborative Group. Effect of allocation to lopinavir–ritonavir or usual care on 28-day mortality. Licensed under CC-BY 4.0.
Association of a prior psychiatric diagnosis with mortality among hospitalized patients with coronavirus disease 2019 (COVID-19) infectionexternal icon. Li et al. JAMA Network Open (September 30, 2020).
Key findings:
- Inpatients with COVID-19 who had a pre-existing psychiatric condition were 1.5 times more likely to die (95% CI 1.1-1.9) during their hospital admission than those without a previous psychiatric diagnosis (Figure).
- 28% of persons admitted had pre-existing psychiatric conditions.
Methods: Cohort study of 1,685 hospitalized patients with COVID-19 admitted between February 15 and April 25, 2020 and followed until May 27, 2020. Mortality rates for patients with and without previously diagnosed psychiatric conditions were compared after adjustment for comorbidities and other potential confounders. Limitations: Limited knowledge of admitting diagnosis and clinical disposition which could affect mortality; no information on previous or current medications.
Implications: Persons hospitalized with COVID-19 with a history of a pre-existing psychiatric conditions were at higher risk of death. Healthcare practitioners need to be vigilant when treating COVID-19 patients who have pre-existing psychiatric conditions.
The length of time a virus survives in the environment may have implications for transmission risk. Here we describe two papers evaluating the survival time of SARS-CoV-2 on human skin and various other surfaces.
PEER-REVIEWED
Survival of SARS-CoV-2 and influenza virus on the human skin: Importance of hand hygiene in COVID-19external icon. Hirose et al. Clinical Infectious Diseases (October 3, 2020).
Key findings:
- SARS-CoV-2 and influenza A virus (IAV) survived on stainless steel, glass, and polystyrene surfaces longer than on skin (>48 hours vs ~2-9 hours).
- Survival time on human skin was ~9 hours for SARS-CoV-2 and ~2 hours for IAV.
- Both SARS-CoV-2 and IAV were completely inactivated on human skin within 15 seconds by treatment with 80% ethanol.
Methods: Survival times of SARS-CoV-2 and IAV on different surfaces and on cadaveric human skin were tested after application in culture media or mucus derived from human sputum. Virus survival time also was evaluated after surface treatment with 80% ethanol. Limitations: One strain of SARS-CoV-2 was investigated; only three skin samples from autopsy specimens were used.
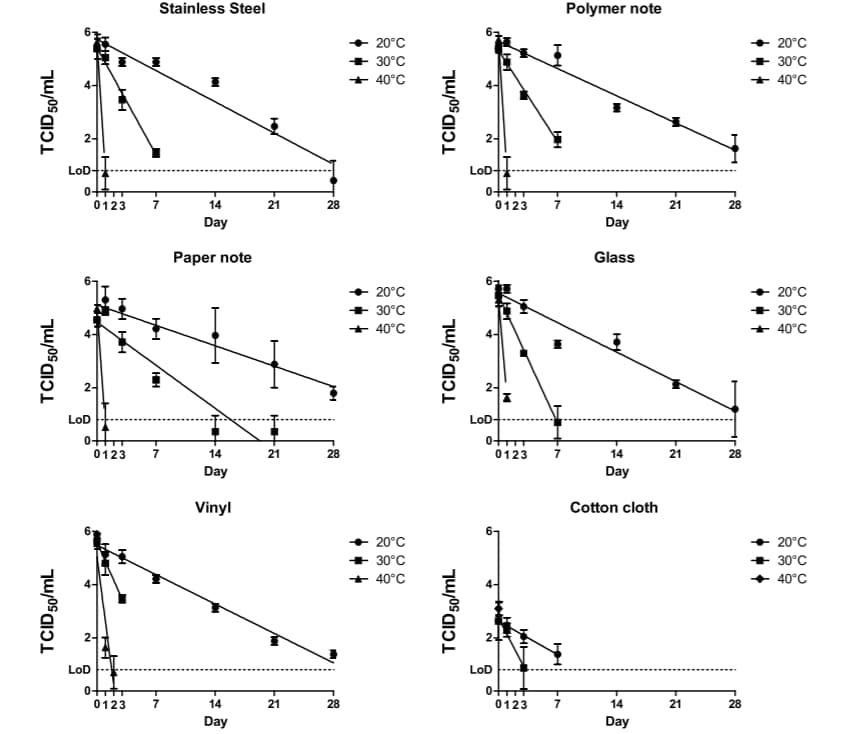
The effect of temperature on persistence of SARS-CoV-2 on common surfaces.external icon Riddell et al. Virology Journal (October 7, 2020).
Key findings:
- The half-life of SARS-CoV-2 on surfaces was between 1.7 and 2.7 days at 20°C and decreased to ≤3 hours at 40°C.
- Infectious SARS-CoV-2 was isolated from all surfaces, except cotton, for up to 28 days at 20°C and up to 2 days at 40°C (Figure).
- Infectious virus was isolated on cotton for up to 14 days at 20°C and up to 1 day at 40°C.
Methods: Survival time of SARS-CoV-2 was tested on various surfaces at 20°C, 30°C, and 40°C with 50% relative humidity. Inoculated samples were kept in the dark for the duration of the experiment. Limitations: Unclear if the study virus titer was that which would reasonably be deposited on surfaces; study was conducted in the dark to remove the effect of UV light, which can inactivate the virus.
Figure:
Note: Adapted from Riddell et al. Recovery of infectious SARS-C0V-2 on surfaces at different temperatures. TCID50, median tissue culture infectious dose at which 50% of cells are infected by the virus; LoD, limit of detection. Licensed under CC-BY.
Implications for 2 studies (Hirose et al. & Riddell et al.): SARS-CoV-2 may have a higher risk of transmission than influenza virus due to longer stability on human skin and ethanol-based disinfectants may be an important intervention to mitigating the risk of transmission. Viable SARS-CoV-2 appears to last for extended periods on various surfaces highlighting the importance of disinfection of surfaces.
Testing
- Rosenberg et al. Widespread and frequent testing is essential to controlling COVID-19 in the United Statesexternal icon. Clinical Infectious Diseases. Editorial arguing that widespread testing is necessary to control the pandemic and highlights effectiveness and cost-effectiveness of various testing scenarios.
- Service, R. A call for diagnostic tests to report viral loadexternal icon. Commentary suggests that diagnostic tests should report not just positive or negative result, but also the cycle threshold value to help identify who is most contagious or at highest risk of severe disease.
Schools
- Walke et al. Preventing and responding to COVID-19 on college campuses. JAMAexternal icon. Outlines the need for expansive testing and rapid isolation and quarantine of potentially infected persons to prevent and respond to outbreaks on college campuses.
- Grech et al. Holidays over: A review of actual COVID-19 school outbreaks up to September 2020. Early Human Developmentexternal icon. A review of school and university outbreaks and social distancing measures.
Clinical Treatment & Management
- Sullivan et al. Cerebrospinal fluid leak after nasal swab testing for coronavirus disease 2019external icon. JAMA Otolaryngology Head & Neck Surgery. Description of the first reported case of a cerebrospinal fluid leak after rupture of an encephalocele with nasal testing for SARS-CoV-2.
- Qaseem et al. Should remdesivir be used for the treatment of patients with COVID-19? Rapid, living practice points from the American College of Physiciansexternal icon (Version 1). Annals of Internal Medicine. Summary of the evidence of the effectiveness and harms of remdesivir and whether these vary by symptom duration, disease severity, and treatment duration; document to be updated every two months.
Vaccine development
- Parker et al. Keeping track of the SARS-CoV-2 vaccine pipelineexternal icon. Nature Reviews Immunology. A brief overview of the online SARS-CoV-2 vaccine development tracker, VaC COVID-19 vaccine trackerexternal icon, that includes information on vaccine candidates, key attributes of all registered SARS-CoV-2 vaccine trials, and a ‘living review’ that summarizes clinical trial results as they become available.
COVID-19 and neurological issues
- Zammit et al. A rise in facial nerve palsies during the coronavirus disease 2019 pandemic. Journal of Laryngology and Otologyexternal icon. Study describing an increase in incidence of facial nerve palsy in 2020 compared to the previous year. Incidence of SARS-CoV-2 was higher among those with facial nerve palsy than in a comparison group.
- Goh et al. Pearls & Oy-sters: Facial nerve palsy in COVID-19 infection. Neurologyexternal icon. Case report of patient with COVID-19 and facial nerve palsy.
- Beauchamp et al. Parkinsonism as a third wave of the COVID-19 pandemic? Journal of Parkinson’s Diseaseexternal icon. Authors propose monitoring persons with history of neurologic symptoms from SARS-CoV-2 infection for potential long-term effects that may include sequelae such as viral-associated parkinsonism.
Miscellaneous
- Balaraman et al. Susceptibility of midge and mosquito vectors to SARS-CoV-2 by natural route. BioRxivexternal icon Studied the susceptibility of biting insects to SARS-CoV-2 after ingesting infected blood meal and concluded that biting insects do not pose a transmission risk to humans or animals.
Disclaimer: The purpose of the CDC COVID-19 Science Update is to share public health articles with public health agencies and departments for informational and educational purposes. Materials listed in this Science Update are selected to provide awareness of relevant public health literature. A material’s inclusion and the material itself provided here in full or in part, does not necessarily represent the views of the U.S. Department of Health and Human Services or the CDC, nor does it necessarily imply endorsement of methods or findings. While much of the COVID-19 literature is open access or otherwise freely available, it is the responsibility of the third-party user to determine whether any intellectual property rights govern the use of materials in this Science Update prior to use or distribution. Findings are based on research available at the time of this publication and may be subject to change.