COVID-19 Science Update released: September 29, 2020 Edition 52

The COVID-19 Science Update summarizes new and emerging scientific data for public health professionals to meet the challenges of this fast-moving pandemic. Weekly, staff from the CDC COVID-19 Response and the CDC Library systematically review literature in the WHO COVID-19 databaseexternal icon, and select publications and preprints for public health priority topics in the CDC Science Agenda for COVID-19 and CDC COVID-19 Response Health Equity Strategy.
Here you can find all previous COVID-19 Science Updates.
PEER-REVIEWED
Infant outcomes following maternal infection with SARS-CoV-2: First report from the PRIORITY studyexternal icon. Flaherman et al. Clinical Infectious Diseases (September 18, 2020).
Key findings:
- In this US study, preterm birth, ICU admission, and respiratory disease did not differ between infants born to mothers testing positive for SARS-CoV-2 and those born to mothers testing negative.
- No pneumonia or lower respiratory tract infection was reported through 6 – 8 weeks of age.
- Infants born to mothers who first tested positive for SARS-CoV-2 ≤14 days prior to delivery were more likely admitted to the ICU (26% vs 12%; p = 0.04) and born earlier (mean 37.5 vs 39 weeks gestation, p = 0.0009) compared with infants born to mothers who tested positive >14 days prior to delivery.
- 6 – 8 weeks postpartum, the estimated incidence of a positive infant SARS-CoV-2 test was 1.1% (95% CI: 0.1 – 4.0).
Methods: Early findings from the PRIORITYexternal icon prospective study among 179 mothers who had a positive test for SARS-CoV-2 and 84 mothers who had a negative test. Data were collected at enrollment, after birth, and at 6 – 8 weeks after delivery. Limitations: May not be representative of all US pregnancies; some associations may reflect hospital practices rather than clinical status; infant testing for SARS-CoV-2 was incomplete.
Implications: Infants born to mothers infected with SARS-CoV-2 generally do well in the first 6 – 8 weeks after birth. Further large-scale studies like PRIORITY need to assess long-term infant and maternal outcomes.
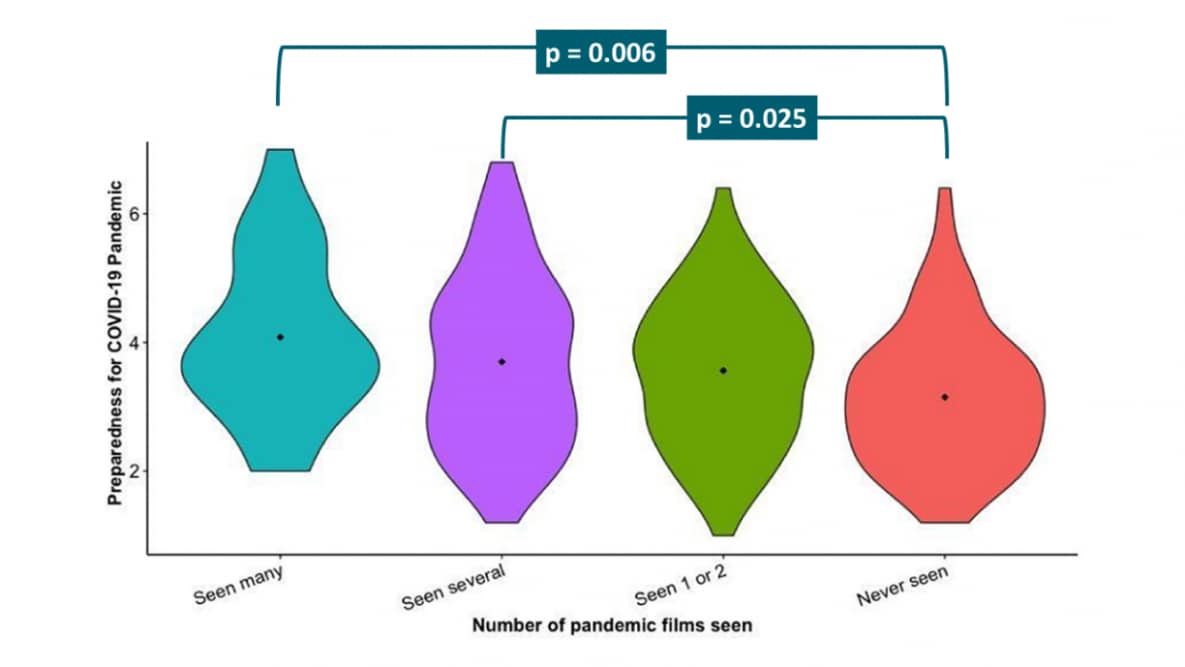
Pandemic practice: Horror fans and morbidly curious individuals are more psychologically resilient during the COVID-19 pandemicexternal icon. Scrivner et al. Personality and Individual Differences (September 15, 2020).
Key findings:
- Horror fandom was significantly associated with lower psychological distress during the current COVID-19 pandemic (p = 0.006) but not with positive resilience or pandemic preparedness.
- Fandom of prepper genres (zombie, apocalyptic, alien-invasion) was associated with lower psychological distress (p = 0.030) and greater COVID-19 pandemic preparedness (p = 0.014).
- Morbid curiosity (a trait that motivates a person to learn about dangerous or threatening phenomena) was significantly associated with positive resilience during the pandemic (p <0.001).
- Watching pandemic films in the past was significantly related to pandemic preparedness (p = 0.003) (Figure).
Methods: 322 US adults recruited and surveyed online in April 2020 with questions on movie genre fandom, COVID-19 pandemic preparedness, pandemic psychological resilience and personality traits. Limitations: Not representative or generalizable; no causality can be inferred; limited confounding covariates.
Implications: Fans of horror or prepper fiction and films and those with morbid curiosity may be more psychologically resilient and prepared during the COVID-19 pandemic. More research would need to be done before recommending engagement with horror or prepper media to increase resilience.
Figure:
Note: Adapted from Scrivner et al. Previous viewing of pandemic films and preparedness scale for the COVID-19 pandemic. This article was published in Personality and Individual Differences, Vol 168, Scrivner et al., Pandemic practice: Horror fans and morbidly curious individuals are more psychologically resilient during the COVID-19 pandemic, Page 110397, Copyright Elsevier 2020. This article is currently available at the Elsevier COVID-19 resource center: https://www.elsevier.com/connect/coronavirus-information-centerexternal icon.
The risk of severe COVID-19 within households of school employees and school-age childrenexternal icon. Selden et al. Health Affairs (September 17, 2020).
Key findings:
- Between 42.0% and 51.4% of all school employees had increased or potentially increased risk of severe COVID-19.
- 58.2% of low-skill support staff were at increased risk, compared with 37.8% of teachers and assistants, and 39.1% of administrators and high-skill support staff.
- Obesity (33.8%) was the primary COVID-19 risk factor.
- 63.2% of school employees and 58.7% of school-aged children lived with ≥1 increased-risk adult.
- 62.1% of high school students vs 55.7% of children 5 – 9 years old lived with increased-risk adults.
- Black (67.3%) and Hispanic (64.6%) children were more likely than White (55.8%) and Asian (35.2%) children to live with increased-risk adults.
- Between 33.9 and 44.2 million adults with direct or within-household connections to schools are potentially at increased risk for severe COVID-19.
Methods: Nationally representative analysis of 95,830 adults from the Medical Expenditure Panel Survey (MEPS),external icon 2014 – 2017. Participants were classified as having increased risk of severe COVID-19 according to CDC guidance. Limitations: Certain medical conditions not included, and results may underestimate risk; self-reported data may undercount obesity; data is from civilian noninstitutionalized population and did not include those in nursing, long-term care, and correctional facilities.
Implications: Plans to reduce COVID-19 risk in schools need to consider actions that minimize risk for large groups of at-risk adults working in school settings and within students’ households.
PEER-REVIEWED
Clustering and superspreading potential of SARS-CoV-2 infections in Hong Kongexternal icon. Adam et al. Nature Medicine (September 17, 2020).
Key findings:
- An investigation of 137 different recognized clusters (median cluster size = 2) found that 7 probable super spreader events (SSEs) accounted for 58% of all clustered cases (Figure).
- The largest cluster of 106 cases was associated with four bars in Hong Kong.
- An estimated 19% (95% CI: 15% – 24%) of cases caused 80% of all local transmission.
- Transmission in social settings was associated with more secondary cases than transmission in households (p = 0.002).
Methods: Contact tracing data from 1,038 confirmed SARS-CoV-2 infections in Hong Kong between January 23 and April 28, 2020 was used to characterize clusters (≥2 cases) of SARS-CoV-2 infections, chains of transmission and description of SSE. Limitations: Potential incomplete identification of cases and contacts; some cases may have been incorrectly attributed to clusters.
Implications: There is substantial potential for SARS-CoV-2 superspreading in settings where large numbers of people gather such as bars, weddings, and religious events. Interventions targeting social settings may be key in reducing the risk of SSEs and SARS-CoV-2 transmission.
Figure:
Note: Adapted from Adam et al. a: Transmission network of the largest SSE (n = 106). b and c: Transmission networks associated with smaller SSEs ([b] n = 22 and [c] n = 19). d: All other clusters of SARS-CoV-2 infections where the source and transmission chain could be determined. Reprinted by permission from Springer Nature Customer Service Centre GmbH: Springer Nature, Nature Medicine, Clustering and superspreading potential of SARS-CoV-2 infections in Hong Kong, Adam et al., COPYRIGHT 2020.
Measurement of SARS-CoV-2 RNA in wastewater tracks community infection dynamicsexternal icon. Peccia et al. Nature Biotechnology (September 18, 2020).
Key findings:
- In New Haven, CT, SARS-CoV-2 RNA in wastewater sludge was identified:
- 0 – 2 days ahead of SARS-CoV-2-positive specimen collection date.
- 1 – 4 days ahead of COVID-19 hospital admissions.
- 6 – 8 days ahead of reporting date of SARS-CoV-2 positive specimens.
Methods: From March 19 through June 1, 2020 in New Haven, Connecticut, SARS-CoV-2 RNA from wastewater sludge was quantitatively measured and 4 COVID-19 epidemiological parameters (SARS-CoV-2 positive test results by date of specimen collection percentage of positive SARS-CoV-2 test results by date of specimen collection number of local hospital admissions of patients with COVID-19 and SARS-CoV-2 positive test results by reporting date) were assessed using Poisson regression models. Limitations: Primary sludge handling approaches vary by treatment plants and could affect levels of detectable virus; epi parameters not fully defined.
Implications: Detecting SARS-CoV-2 in wastewater sludge can provide advance notice and could possibly act as an early warning system of infections in the community served by the sewage system.
PEER-REVIEWED
No SARS-CoV-2 neutralization by intravenous immunoglobulins produced from plasma collected before the 2020 pandemic. external iconSchwaiger et al. Journal of Infectious Diseases (September 17, 2020).
Key findings:
- Among 54 pre-pandemic intravenous immunoglobulin (IVIG) products tested, all neutralized a seasonal human coronavirus (HCoV-229E), while SARS-CoV-2 neutralizing antibodies (NAbs) were not detected in any product (Figure).
- Testing of post-pandemic plasma samples indicated that up to 1.17% of plasma donors were positive for SARS-CoV-2 NAbs.
Methods: 54 IVIG pools of plasma donations from plasmapheresis (source, S) or recovered from whole blood donations (recovered, R) prior to the circulation of SARS-CoV-2 in the US (n = 30) and central Europe (n = 24) were tested for NAbs to HcoV-229E and SARS-CoV-2. In addition, 560 plasma pools of six donations each from March to July 2020 in Austria were tested for NAbs. Limitations: No testing for binding antibodies which may have other functional properties.
Implications: The absence of cross-reactive antibodies against SARS-CoV-2 from pre-pandemic plasma shows that currently available IVIGs cannot afford protection from SARS-CoV-2 infection. Presence of convalescent NABs is necessary in plasma-derived immune products to be used as a potential treatment option for COVID-19.
Figure:
Note: Adapted from Schwaiger et al. Pre-pandemic IVIG lots from recovered [R] or source [S] blood in US and Europe tested for NAbs to SARS-CoV-2. Licensed under CC-BY-NC-ND.
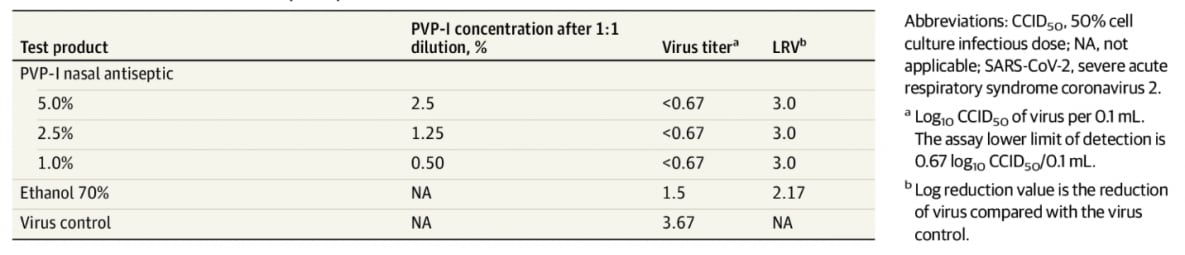
In vitro efficacy of a povidone-iodine nasal antiseptic for rapid inactivation of SARS-CoV-2external icon. Frank et al. JAMA Otolaryngology – Head & Neck Surgery (September 17, 2020).
Key findings:
- Povidone-iodine nasal antiseptic (PVP-I) inactivated SARS-CoV-2 within 15 seconds in vitro (Table).
Methods: Controlled in vitro study of SARS-CoV-2 virus tested against 3 concentrations of PVP-I (0.5%, 1.25%, and 2.5%) for 15 seconds and 30 seconds. Limitations: in vitro study; safety and efficacy of intranasal PVP-I not known.
Implications: Nasal decontaminants have been advocated as a way to sterilize the nasal cavity in persons with SARS-CoV-2 infection, as well as for prophylaxis in exposed persons to reduce transmission and to diminish the severity of disease. These early results demonstrate effectiveness of PVP-I against SARS-CoV-2 in vitro. More comprehensive studies should be undertaken to examine the safety and potential in vivo effectiveness of PVP-I as a possible measure to mitigate viral transmission.
Table:
Note: Adapted from Frank et. al. SARS-CoV-2 tested against 3 concentration of PVP-I (0.5%, 1.25%, and 2.5%) for 15 seconds. Ethanol 70% was used as a positive control and did not completely inactivate SARS-CoV-2 after 15 seconds of contact. Virus control contained no antiseptic. Reproduced with permission from JAMA Otolaryngology: doi:10.1001/jamaoto.2020.3053. Copyright©2020 American Medical Association. All rights reserved.
PEER-REVIEWED
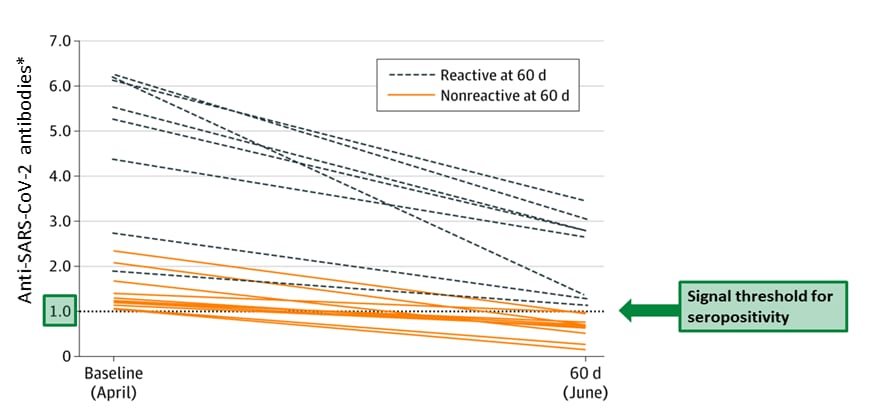
Change in antibodies to SARS-CoV-2 over 60 days among health care personnel in Nashville, Tennesseeexternal icon. Patel et al. JAMA (September 17, 2020).
Key findings:
- Overall seropositivity of anti-SARS-CoV-2 antibodies among healthcare personnel (HCP) decreased from 7.6% at baseline (April 2020) to roughly 3.2% approximately 60 days later.
- 58% of 19 seropositive individuals became seronegative (Figure).
- 6 of 8 participants who remained seropositive reported symptoms prior to the baseline visit, and 2 of 8 were asymptomatic.
- All baseline seropositive HCP had declines in antibody titers (Figure).
- Participants who remained seropositive at 60 days had higher antibody levels at baseline (mean 4.8 signal-to-threshold ratio) compared with participants who became seronegative (mean 1.4).
Methods: Antibody levels were assessed in a convenience sample of 249 HCP at baseline (April 3 to April 13, 2020) and approximately 60 days later (June 2 to June 27, 2020) in Nashville, Tennessee. Specimen were considered reactive at a signal-to-threshold ratio indicating ratios >1, with higher ratios having higher antibody titers. Limitations: Small sample size and not generalizable; timing of infection uncertain.
Implications: HCP with previous infection showed declines in antibody levels, could be at risk of reinfection, and should adhere to proper infection control guidelines.
Figure:
Note: Adapted from Patel et al. Anti-SARS-CoV-2 spike antibody levels in 19 seropositive HCP at baseline, (April) and 60 days (June). Dotted line at 1.0 and green arrow indicates the threshold for seropositivity. *Using signal-to-threshold ratio.
Reproduced with permission from JAMA: doi:10.1001/jama.2020.18796. Copyright©2020 American Medical Association. All rights reserved.
SARS-CoV-2 Testing
- Guglielmi G. Fast coronavirus tests: What they can and can’t doexternal icon. Nature News Feature. Describes different types of tests available for SARS-CoV-2 detection: RT-PCR tests, rapid antigen tests and antibody tests.
- Gibani et al. Assessing a novel, lab-free, point-of-care test for SARS-CoV-2 (CovidNudge): A diagnostic accuracy studyexternal icon. Lancet Microbe. A rapid point of care PCR test (COVIDNudge) reports high sensitivity (94%) and specificity (100%) compared with an RT-PCR standard.
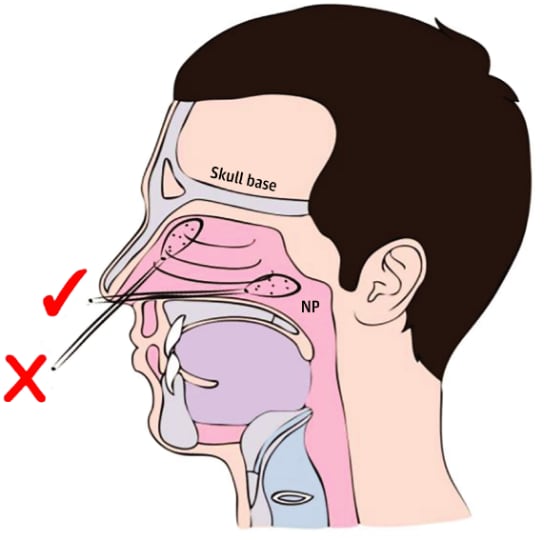
- Higgins et al. SARS-CoV-2 nasopharyngeal swab testing — False-negative results from a pervasive anatomical misconceptionexternal icon. JAMA Otolaryngology-Head & Neck Surgery. Improper technique resulting in swabs not reaching the nasopharynx might be a source of false-negative results.
Note: From Higgins et al. Diagram of nasal anatomy showing the correct (✓) and incorrect (X) trajectory for a swab directed into the nasopharynx (NP). Reproduced with permission from JAMA Otolaryngology: doi:10.1001/jamaoto.2020.2946. Copyright©2020 American Medical Association. All rights reserved.
Transmission
- Meyerowitz et al. Transmission of SARS-CoV-2: A review of viral, host, and environmental factorsexternal icon. Annals of Internal Medicine. Reviews the evidence on modes of transmission of SARS-CoV-2.
Immunity
- Huang et al. A systematic review of antibody mediated immunity to coronaviruses: Kinetics, correlates of protection, and association with severityexternal icon. Nature Communications. Reviews the scientific literature on antibody measures of immunity to SARS-CoV-2, SARS-CoV, MERS-CoV and endemic human coronaviruses.
- Jones et al. A history of herd immunityexternal icon. Lancet. Provides a detailed look into how the term was coined and the history of the concept in public health.
Case Reports
- Larson et al. A case of early re-infection with SARS-CoV-2external icon. Clinical Infectious Diseases. An immunocompetent healthcare provider with mild symptoms tested positive for SARS-CoV-2 and tested positive again 51 days after resolution of initial infection.
- A case of probable Parkinson’s disease after SARS-CoV-2 infectionexternal icon. Lancet Neurology. Report of probable Parkinson’s disease in a patient after SARS-CoV-2 infection.
Social Aspects
- Tan et al. Location Matters: Geographic disparities and impact of coronavirus disease 2019 (COVID-19)external icon. Journal of Infectious Disease. Highlights racial, ethnic, locale, and socioeconomic disparities of the COVID-19 outbreak in the US and presents recommendations on how to ameliorate the current situation.
- Cahan et al. The human touch — Addressing health care’s workforce problem amid the pandemicexternal icon. NEJM. Several strategies to improve and enhance the healthcare response including coordinating and deploying a volunteer workforce, and loosening state-level licensing restrictions for physicians and nonphysician providers.
Disclaimer: The purpose of the CDC COVID-19 Science Update is to share public health articles with public health agencies and departments for informational and educational purposes. Materials listed in this Science Update are selected to provide awareness of relevant public health literature. A material’s inclusion and the material itself provided here in full or in part, does not necessarily represent the views of the U.S. Department of Health and Human Services or the CDC, nor does it necessarily imply endorsement of methods or findings. While much of the COVID-19 literature is open access or otherwise freely available, it is the responsibility of the third-party user to determine whether any intellectual property rights govern the use of materials in this Science Update prior to use or distribution. Findings are based on research available at the time of this publication and may be subject to change.

![929_FIG2 Transmission network of the largest SSE (n = 106). b and c: Transmission networks associated with smaller SSEs ([b] n = 22 and [c] n = 19). d: All other clusters of SARS-CoV-2 infections where the source and transmission chain could be determined.](/library/covid19/images/929_FIG2.png?_=93903)
![929_FIG3 Pre-pandemic IVIG lots from recovered [R] or source [S] blood in US and Europe tested for NAbs to SARS-CoV-2.](/library/covid19/images/929_FIG3.png?_=94415)