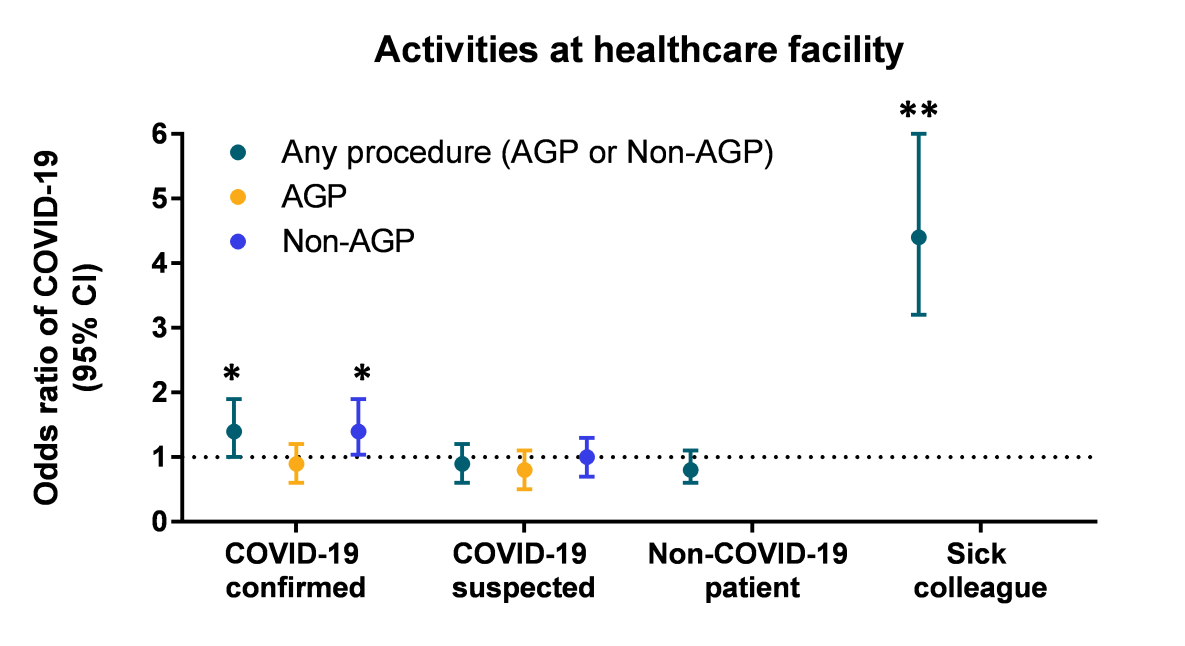COVID-19 Science Update released: September 18, 2020 Edition 49

The COVID-19 Science Update summarizes new and emerging scientific data for public health professionals to meet the challenges of this fast-moving pandemic. Weekly, staff from the CDC COVID-19 Response and the CDC Library systematically review literature in the WHO COVID-19 databaseexternal icon, and select publications and preprints for public health priority topics in the CDC Science Agenda for COVID-19 and CDC COVID-19 Response Health Equity Strategy.
Here you can find all previous COVID-19 Science Updates.
Below we present three studies that investigate the risks for infection with SARS-CoV-2 in healthcare settings. These studies look at occupational as well as nosocomial risks for patients and healthcare workers (HCWs). Across all three studies, higher levels of infection control and prevention protect both patients and HCWs.
PEER-REVIEWED
A. SARS-CoV-2 seroprevalence and asymptomatic viral carriage in healthcare workers: A cross-sectional studyexternal icon. Shields et al. Thorax (September 11, 2020).
Key findings:
- Over a 24-hour period, 2.4% (n = 13/545) of asymptomatic HCWs tested RT-PCR positive.
- The seroprevalence among HCWs was 4 times higher than reported regionally (24% vs 6%).
- Black, Asian, and minority ethnicity HCWs were at increased risk for seropositivity (adjusted OR: 1.92, 95% CI 1.14 to 3.23, p = 0.01), even after controlling for external risk factors.
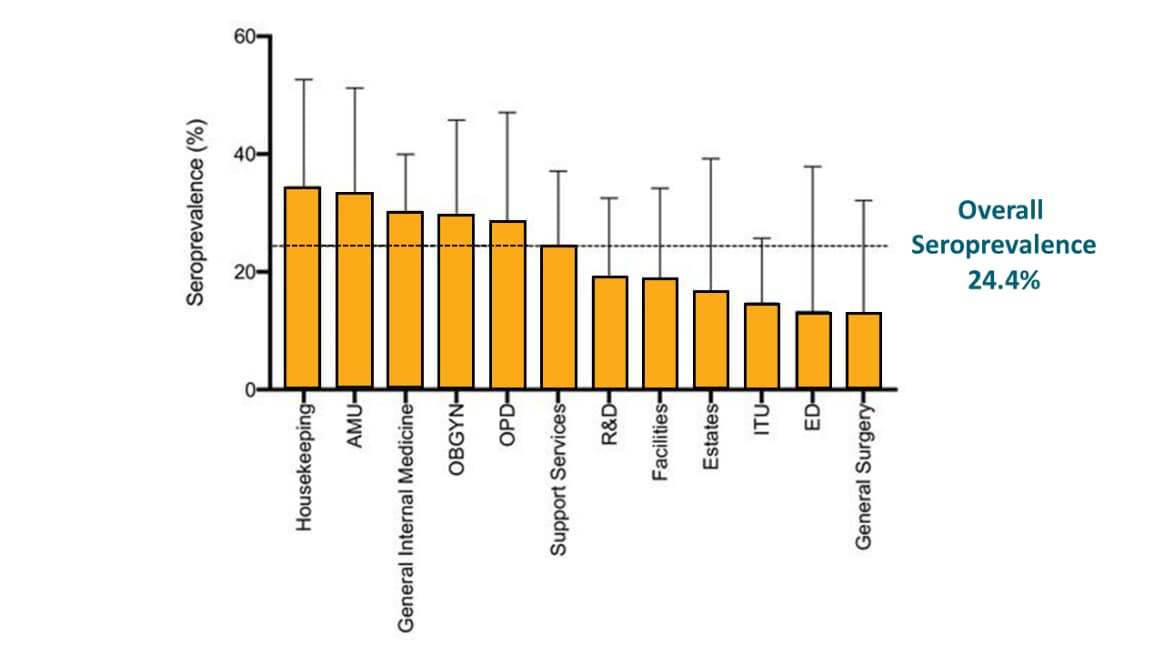
- Seropositivity was highest among housekeeping, acute medicine, and general internal medicine staff and lowest among intensive care, emergency department, and general surgery staff (Figure).
Methods: Cross-sectional convenience sample of 545 asymptomatic HCWs recruited at work in Birmingham, UK, on April 24 and April 25, 2020. Anyone with symptoms of COVID-19 on that day, who was home due to self-isolation, or had symptomatic illness was excluded. Participants were tested using RT-PCR and serology. Limitations: The study did not look at how representative the study respondents were of the worker cohort at the time of the study; single cross-sectional sampling may underestimate the seroprevalence.
Figure:
Note: Adapted from Shields et al. Seroprevalence of SARS-CoV-2 antibody by department. AMU-acute medical unit; ED-emergency department; ITU-intensive care unit; OBGYN-obstetrics and gynecology; OPD-outpatient department; R&D-research and development. Licensed under CC-BY-NC 4.0.
B. Assessing COVID-19 Transmission to Healthcare Personnel: The Global ACT-HCP Case-Control Studyexternal icon. Lentz et al. Infection Control and Hospital Epidemiology (September 9, 2020).
Key findings:
- The proportion of COVID-19 cases was greater among nurses (41%) than either physicians and nurse practitioners (20%) or respiratory therapists (6%).
- At work, contact with a sick colleague, contact with patients with laboratory-confirmed COVID-19, and performing non-aerosol-generating procedures (AGP) on patients with laboratory-confirmed or suspect COVID-19 were significantly associated with testing positive for SARS-CoV-2 (Figure 1).
- Outside of work, attending gatherings of >10 people, going to a restaurant or bar, using public transportation, or being exposed to a household member with COVID-19 were significantly associated with testing positive for SARS-CoV-2 (Figure 2).
Methods: Case-control study among 1,678 healthcare personnel from 67 countries using an online survey tool, between April 20 and May 5, 2020. Inclusion criteria included working in a healthcare setting between January 1, 2020 and survey completion. Cases (n = 244) were those reporting a laboratory-confirmed COVID-19 diagnosis and controls (n = 886) were those who remained healthy. Possible cases who experienced COVID-19 symptoms without laboratory confirmation were excluded. Limitations: Mid-level providers and physicians were overrepresented; confirmation of case or control status was not possible; asymptomatic cases may have been present in the control group.
Figure 1
Note: Adapted from Lentz, et al. OR of COVID-19 in healthcare workers based on type of contact with patients and colleagues for Any procedure data, AGP and Non-AGP. Bars represent the 95% CI and the dashed line is OR of 1, PUI-person under investigation, *p-value <0.05, **p-values <0.01. Reproduced with permission of Cambridge University Press.
Figure 2
Note: Adapted from Lentz et al. ORs for COVID-19 in healthcare workers based on activities outside of work. Dots represent the ORs, while bars represent 95% CI. **p-value <0.01, ***p-value <0.001. Reproduced with permission of Cambridge University Press.
C. Incidence of nosocomial COVID-19 in patients hospitalized at a large US academic medical centerexternal icon. Rhee et al. JAMA Network Open (September 9, 2020).
Key findings:
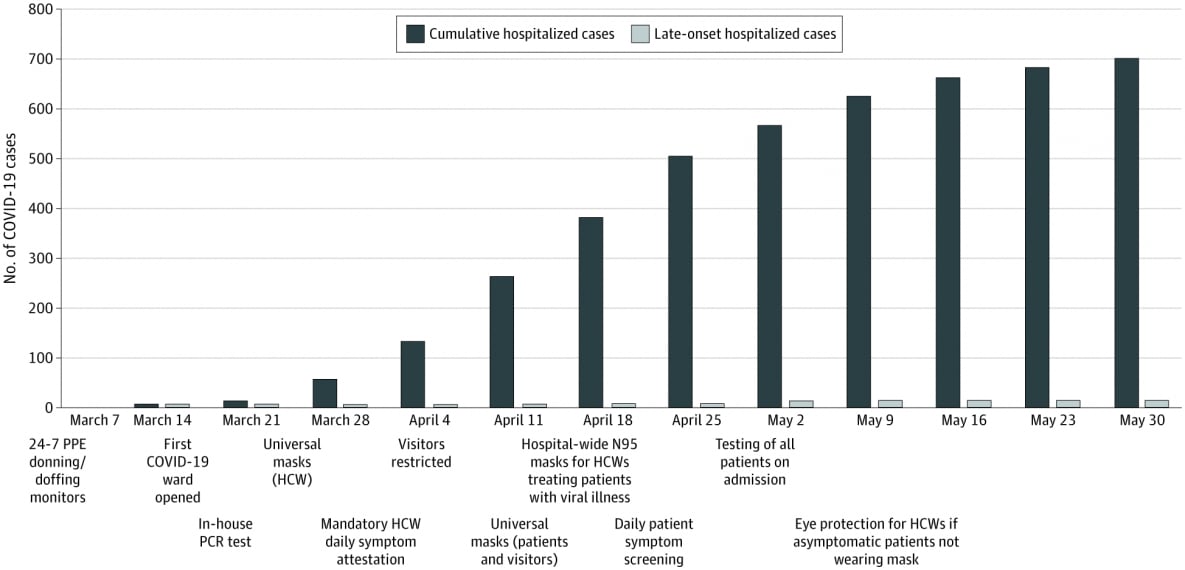
- 12/697 (1.7%) hospitalized patients tested positive for SARS-CoV-2 infection after admission.
- Only 1 patient acquired SARS-CoV-2 infection while hospitalized through exposure to a visiting asymptomatically infected spouse.
- 11/8,370 (0.1%) patients tested positive for SARS-CoV-2 infection within 14 days of discharge.
- In one person, infection was likely hospital-acquired.
Methods: Cohort study among all 9,149 patients admitted to Brigham and Women’s Hospital in Boston, MA, between March 7 and May 30, 2020. SARS-CoV-2 infection was determined by positive RT-PCR. Test timing, clinical course, and exposure history were used to classify cases as having community- or hospital-acquired SARS-CoV-2 infection. Limitations: Testing practices changed during the study period; asymptomatic cases might not have sought testing after discharge; those who may have sought testing more than 14 days after discharge and cases diagnosed outside the hospital catchment area were not captured; the hospital did not exceed surge capacity during the study period.
Figure:
Note: Adapted from Rhee et al. Cumulative number of Total and Late-Onset hospitalized coronavirus disease 2019 (COVID-19) cases by week. Licensed under CC-BY.
Implications for 3 studies (Shields et al., Lentz et al. & Rhee et al.): Healthcare settings present risks for SARS-CoV-2 infection to both healthcare personnel and patients. Healthcare personnel face different risks based on occupation and afterwork activities. With comprehensive infection control programs, risk of nosocomial and healthcare-associated SARS-CoV-2 infection among patients and staff, respectively, can be minimized even in the context of high-risk procedures and settings.
PEER-REVIEWED
A. COVID‐19 death rates are higher in rural counties with larger shares of Blacks and Hispanicsexternal icon. Cheng et al. Journal of Rural Health (September 7, 2020).
Key findings:
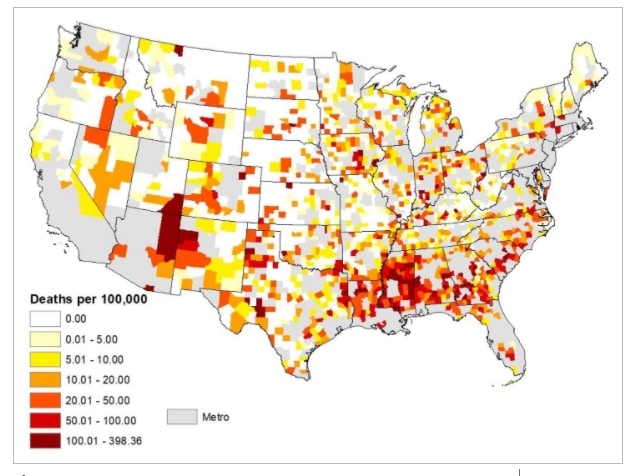
- The average daily increase in COVID‐19 mortality has been significantly greater in rural counties with the largest percentages of Black and Hispanic residents (Figure).
- In this study, when the 20 rural counties with the highest mortality rates were stratified in quartiles by percentage of racial/ethnic minority residents:
- By Black race, the average daily increase in COVID-19 deaths was 70% higher in the top quartile compared with the bottom quartile (incidence rate ratio (IRR) 1.70, CI 1.48-1.95, p <0.001).
- By Hispanic ethnicity, the average daily increase in COVID-19 deaths was 50% higher in the top quartile compared with the bottom quartile (IRR 1.50, CI 1.33-1.69, p <0.001).
Methods: Regression analysis was used to measure differences in the increase in the COVID-19 mortality rate based on the proportion of the Black or Hispanic population during the first 5 months of the pandemic from 1,976 US non-metropolitan US counties. Limitations: This study did not assess individual mortality risk; because race/ethnicity-specific COVID-19 mortality data were lacking, it’s possible that White persons also had higher mortality rates in rural areas.
Figure:
Note: From Cheng et al. COVID-19 mortality rates (per 100,000 persons) in non-metro counties. Gray counties are metro counties. Permission request in process. Reproduced by permission of John Wiley and Sons. © 2020 National Rural Health Association.
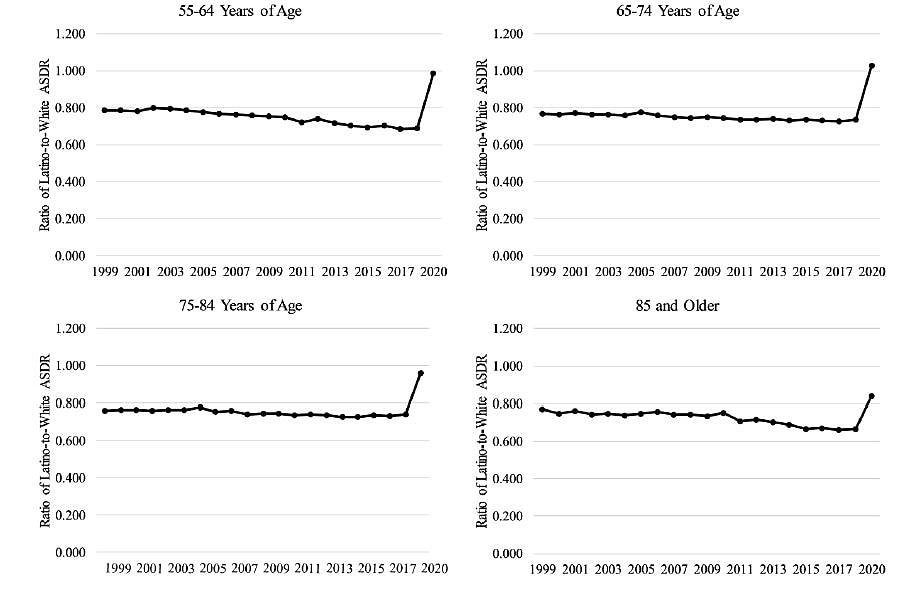
B. The disproportionate impact of COVID-19 on older Latino mortality: The rapidly diminishing Latino paradox. external iconSaenz et al. Journal of Gerontology (September 8, 2020).
Key findings:
- Historically, age-specific death rates (ASDR) for Hispanic persons have been lower than for Whites. However, in 2020 the death rates for Hispanics increased in all age groups (Figure).
- Older Hispanic persons experienced a higher COVID-19 age-adjusted mortality than White persons across all age groups; however, Hispanic persons had lower non-COVID-19 age-adjusted mortality than White and Black persons.
- The difference in COVID-19 age-adjusted mortality decreased in older age groups (i.e., 6.1 times higher in Hispanic persons compared with White persons in the 55-64 years age group vs 1.6 times higher than Whites persons in the ≥85 years age group).
Methods: Investigators estimated age-adjusted mortality rates from February to August, 2020 and the age-adjusted mortality ratio of Hispanic persons to White persons across four age groups (55-64 yrs., 65-74 yrs., 75-84 yrs., and 85+). Mortality rates from 1999 to 2018 were used for historical comparisons. Limitations: Mortality data were provisional and subject to revision.
Figure:
Note: From Saenz et al. Age-specific death ratios in Hispanic persons compared with White persons, 1999-2020. Values lower than 1 indicate a lower death rate for Hispanic persons relative to White persons. Permission request in process. Reproduced by permission of Oxford University Press on behalf of the Gerontological Society of America. Please visit: https://academic.oup.com/psychsocgerontology/advance-article/doi/10.1093/geronb/gbaa158/5902962external icon
Implications of both studies (Cheng et al. & Saenz et al.): Communities of color bear a disproportionate share of the mortality risk of COVID-19 and this extends to rural areas of the US, highlighting the need to address structural inequities that contribute these mortality differences. Greater COVID-19 age-adjusted mortality in Hispanic persons relative to White persons has diminished the “Latino paradox” in which Latino persons have historically had greater longevity than non-Hispanic persons.
PEER-REVIEWED
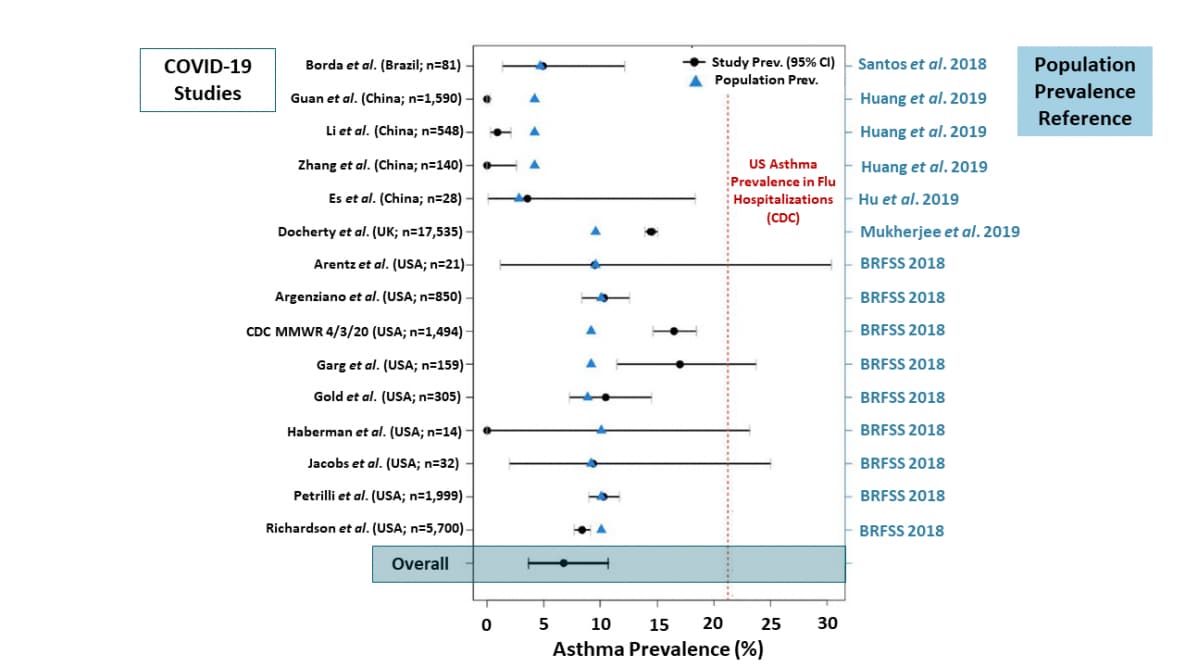
Asthma in COVID-19 hospitalizations: An overestimated risk factor?external icon Broadhurst et al. Annals of the American Thoracic Society (August 31, 2020).
Key findings:
- Asthma prevalence among hospitalized COVID-19 patients is not greater than that of the local population and lower than US hospitalized influenza patients (6.8% pooled estimate vs 21%) (Figure).
- Hospitalized COVID-19 patients with asthma were not more likely to be intubated than COVID-19 patients without asthma (OR 0.69, 95% CI 0.33-1.45, adjusting for age, sex, and body mass index).
Methods: Meta-analysis conducted among 15 studies comparing asthma prevalence among COVID-19 hospitalized patients to local population prevalence and asthma prevalence among US influenza hospitalizations. Additionally, the likelihood of intubation among asthmatics was evaluated among 436 hospitalized COVID-19 patients in one hospital in Colorado. Limitations: Possible difference in comorbidity reporting and four studies added after initial review for meta-analysis; small sample size and limited generalizability for cross-sectional study.
Implications: Asthma does not appear to be a significant risk factor for hospitalization or intubation from COVID-19.
Figure:
Notes: Adapted from Broadhurst et al. Asthma prevalence in various COVID-19 studies. Dots are asthma prevalence among COVID-19 hospitalized patients with 95% CI in 15 studies and triangles are the corresponding population asthma prevalence. The vertical dotted line is the 4-year average US asthma prevalence among influenza hospitalizations. Licensed under CC-BY-NC-ND 4.0.
PEER-REVIEWED
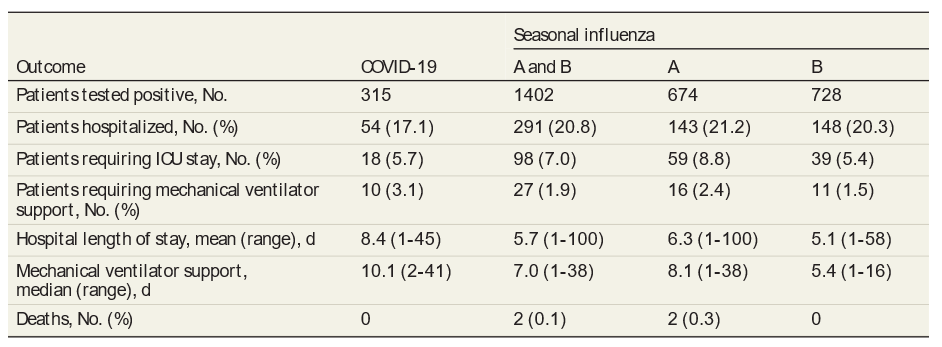
Comparison of clinical features of COVID-19 vs seasonal influenza A and B in US children.external icon Song et al. JAMA Network Open (September 8, 2020).
Key findings:
- There was no significant difference in clinical outcomes between children with COVID-19 versus seasonal influenza with regard to hospitalization (17% vs 21%), ICU admission (6% vs 7%) and mechanical ventilation (3% vs 2%) (Table).
- More COVID-19 patients had fever, diarrhea or vomiting, myalgia and chest pain than patients with seasonal influenza.
- 65% of patients hospitalized for COVID-19 had at least one comorbidity compared with 42% of patients hospitalized for seasonal influenza (p = 0.002).
Methods: Retrospective cohort study comparing 315 children diagnosed with COVID-19 between March 25 and May 15, 2020 and 1,402 children diagnosed with seasonal influenza between October 1, 2019 and June 6, 2020 at Children’s National Hospital in Washington D.C. Diagnosis of either COVID-19 or influenza was confirmed by RT-PCR. Clinical outcomes and symptoms were compared. Limitations: Retrospective study with potential recall bias and missing information; single hospital; some patients were over the age 20 years.
Implications: Clinical outcomes appeared similar comparing children with COVID-19 with those who had seasonal influenza. Diagnosis and clinical management of children with acute respiratory infections may be challenging without diagnostic testing for influenza and SARS-CoV-2.
Table:
Note: Adapted from Song et al. Outcomes in pediatric patients with COVID-19 or seasonal influenza. Numbers in parentheses represent either the percentage or range (in days). Licensed under CC-BY.
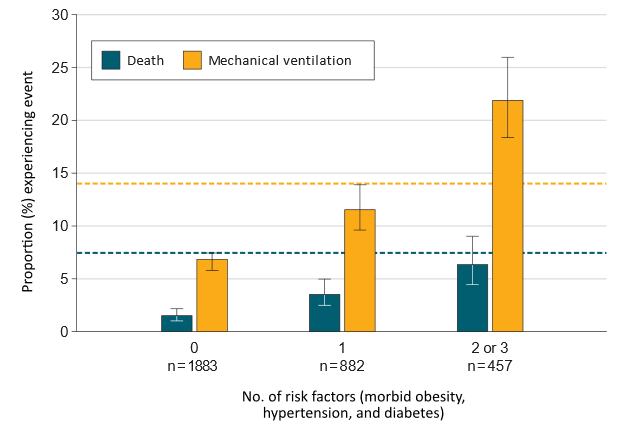
Clinical outcomes in young US adults hospitalized with COVID-19external icon. Cunningham et al. JAMA Internal Medicine (September 9, 2020).
Key findings:
- Of 3,222 adults age 18-34 years, 684 patients (21%) required intensive care; 331 (10%) required mechanical ventilation and 88 (2.7%) died.
- A higher proportion of young adults with 2 or 3 comorbid conditions (either morbid obesity, hypertension, or diabetes) required mechanical ventilation or died compared with young adults with no comorbidities.
- The proportion of young adults with 2 or 3 comorbidities requiring mechanical ventilation was greater than the proportion of older adults with no comorbidities (Figure).
- Proportions of deaths were similar between these two groups.
Methods: Evaluation of adults with COVID-19 hospitalized between April 1 and June 30, 2020. Outcomes of 3,222 adults aged 18-34 years were compared with 8,862 adults age 35-64 years. Limitation: ICD-10 coding might have resulted in misclassification; no confirmation of laboratory diagnosis of SARS-CoV-2 infection.
Implications: Young adults are at risk for severe COVID-19 and death. The increased risk for mechanical ventilation and death conferred by multiple comorbidities common among young persons is comparable to that of otherwise healthy older adults.
Figure:
Note: Adapted from Cunningham et al. Number of risk factors (morbid obesity, hypertension, diabetes) and proportion of death and mechanical ventilation in persons 18-34 years. Dashed lines are proportion of persons age 35-64 years with COVID-19 and no comorbidities who required mechanical ventilation or died. Reproduced with permission from JAMA Intern Med. doi:10.1001/jamainternmed.2020.5313external icon. Copyright©2020 American Medical Association. All rights reserved.
Echocardiographic Findings in Pediatric Multisystem Inflammatory Syndrome Associated with COVID-19 in the United States.external icon Matsubara et al. Journal of American College of Cardiology. (September 2, 2020).
Key findings:
- One (4%) of 28 patients with pediatric multisystem inflammatory syndrome associated with COVID-19 (MIS-C) had coronary artery abnormalities that resolved on follow up; in the Kawasaki disease (KD) group, 4 (20%) of 20 patients had coronary artery abnormalities.
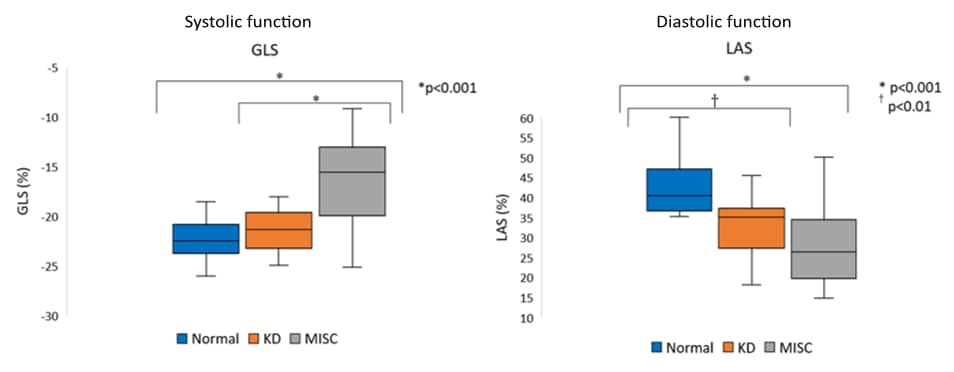
- Compared with healthy children and children with KD, 61% of children with MIS-C had weaker heart function (left ventricular systolic and diastolic function) (Figure 1).
- This impaired function was associated with myocardial injury (myocarditis).
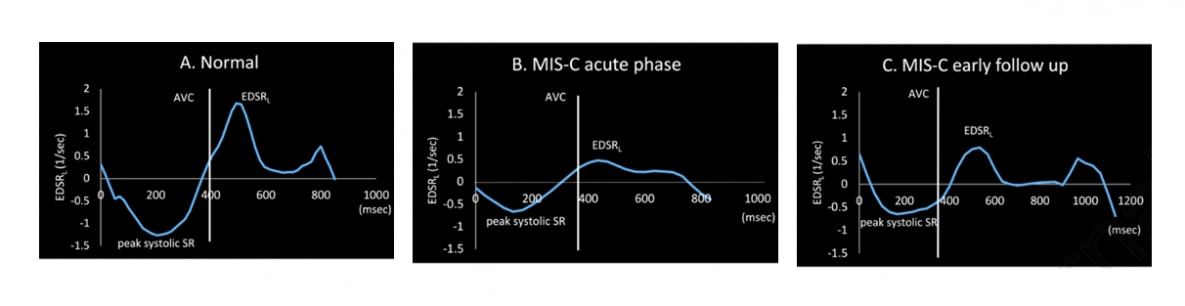
- During the early follow-up period (the period after the acute period), there was good recovery of systolic (pumping) function but diastolic (relaxation) dysfunction persisted compared with the acute phase (Figure 2).
Methods: Retrospective single-center study at the Children’s Hospital of Philadelphia, of 28 pediatric patients with MIS-C caused by SARS-CoV-2 infection, compared with 20 KD patients, and 20 age-matched healthy controls. Echocardiographic imaging and laboratory data were reviewed in acute phase of MIS-C and KD groups, and during early follow-up period in MIS-C group (interval: 5.2 ± 3 days). Limitations: Small sample of MIS-C; short follow up period; MIS-C patients were significantly older and had larger statures than the KD group; no endomyocardial biopsy or cardiac magnetic resonance imaging performed.
Implications: The study found MIS-C caused by SARS-CoV-2 infection was accompanied by changes in myocardial function that differs from what is seen in KD. Early recognition and diagnosis of MIS-C to more accurately assess cardiac function will aid prompt treatment and better outcomes. Long term effects of MIS-C are currently unknown.
Figure 1
Note: Adapted from Matsubara et al. Decreases in systolic and diastolic function in patients with MIS-C. Increased global longitudinal strain (GLS) indicates decrease in systolic function in patients with KD and MIS-C. Decreased peak left atrial strain (LAS) shows decreased diastolic function in patients with KD and MIS-C.
Figure 2
Note: Adapted from Matsubara et al. Early diastolic strain rate curves in a normal (A), MIS-C patient during acute phase (B), and MIS-C patient during early follow up period (C). Reduced EDSR in acute phase resolves during the follow up but remains lower compared with normal. AVC-aortic valve closure; EDSR-early diastolic strain rate; SR-strain rate. This article was published in Journal of American College of Cardiology, Vol 76, Matsubara et al., Echocardiographic findings in pediatric multisystem inflammatory syndrome associated with COVID-19 in the United States, Page 1947-1961, Copyright Elsevier on behalf of American College of Cardiology Foundation 2020. This article is currently available at the Elsevier COVID-19 resource center: https://www.elsevier.com/connect/coronavirus-information-centerexternal icon.
Adverse outcomes and mortality in users of non-steroidal anti-inflammatory drugs who tested positive for SARS-CoV-2: A Danish nationwide cohort studyexternal icon. Lund et al. PLOS Medicine (September 8, 2020).
Key findings:
- Use of non-steroidal anti-inflammatory drugs (NSAID)s for treatment of symptoms related to COVID-19 infection was not associated with an increased risk of 30-day mortality.
- The NSAID user group had a mortality of 6.3%, (95% CI: 3.1% to 9.4%) while non-users had a mortality of 6.1% (95% CI 4.4% to 7.8%).
- The NSAID user group did not have an increased risk of hospitalization, ICU admission, mechanical ventilation, or renal replacement therapy compared with the non-users group.
Methods: Population-based cohort study of all individuals who tested positive for SARS-CoV-2 by RT-PCR between February 27 and April 29, 2020 in Denmark. Infected individuals were grouped according to those who did (n = 248) or did not (n = 8,988) have a prescription filled for NSAIDs up to 30 days prior to diagnosis. Limitations: Potential misclassification of non-users and users as it is not known if NSAIDs were taken.
Implications: It is reassuring that NSAIDs do not appear to lead to a more severe course of COVID-19 as this class of drugs offers benefit in the treatment of constitutional symptoms associated with SARS-CoV-2 infection.
Azithromycin in addition to standard of care versus standard of care alone in the treatment of patients admitted to the hospital with severe COVID-19 in Brazil (COALITION II): A randomised clinical trial.external icon Furtado et al. Lancet (September 4, 2020).
Key findings:
- There was no significant difference in clinical status at hospital day 15 between the persons treated with azithromycin plus standard care and those treated with standard care only (OR 1.36, 95% CI 0.94 –1.9, p = 0.11).
- There was no significant difference in 29-day mortality between the azithromycin and the standard care-only groups: 90 deaths (42%) vs 73 deaths (40%), respectively (hazard ratio 1.08, 95% CI 0.79-1.47, p = 0.63).
Methods: Randomized, open-label, multi-center trial at 57 centers in Brazil. Patients with severe COVID-19 received azithromycin (500 mg once daily for 10 days) with standard care (n = 214) or standard care only (n = 183). Standard care included hydroxychloroquine for 10 days. The primary endpoint was clinical status at day 15 and a secondary outcome was mortality at day 29. Limitations: The protocol was revised four times during the trial regarding entry criteria and analysis; only severe COVID-19 cases included, precluding any effects in milder cases; study did not examine azithromycin as a standalone therapy.
Implications: This study does not support the routine use of azithromycin in patients with severe COVID-19.
PEER-REVIEWED
Broad and strong memory CD4+ and CD8+ T cells induced by SARS-CoV-2 in UK convalescent individuals following COVID-19.external icon Peng et al. Nature Immunology (September 4, 2020).
Key findings:
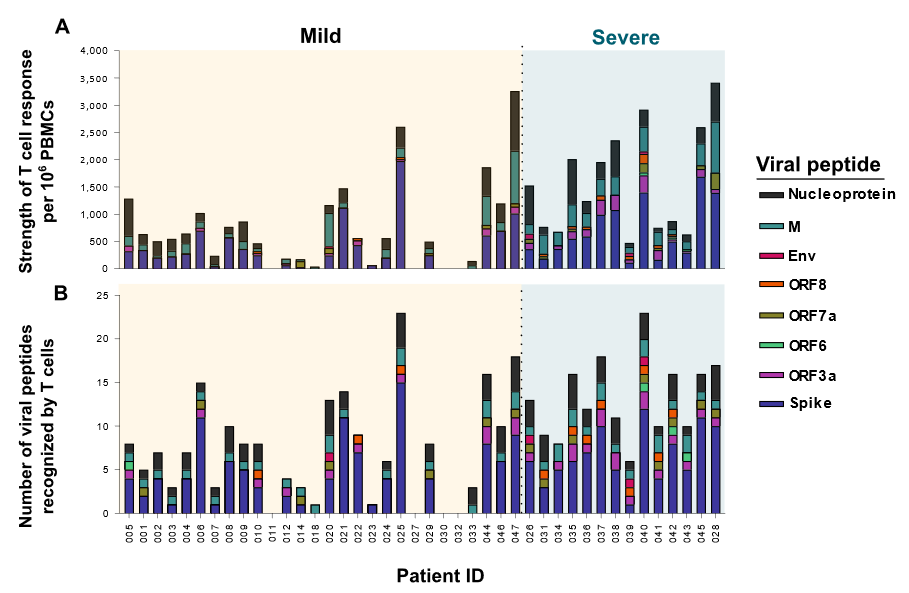
- T cell responses were significantly higher in persons with severe COVID-19 as compared with persons with mild COVID-19 (Figure).
- T cells from persons with severe COVID-19 recognized more viral epitopes than those from persons with mild disease.
Methods: A total of 42 persons recovered from COVID-19 were enrolled between March and May 2020 and classified as either mild (n = 28) or severe cases (n = 14). Specimens from 16 individuals collected between 2017 and 2019 were used as controls. Peripheral blood mononuclear cells (PBMCs) were exposed to pools of peptides spanning the SARS-CoV-2 genome. Limitations: Excluded analysis of peptides from ORF 1ab of SARS-CoV-2, which is more than 2/3 of the genome.
Implications: Persons recovered from COVID-19 possess strong and broad T cell responses potentially indicative of long-lasting protective immunity. The broad response to several different epitopes suggests vaccine-escape viruses would need several mutations.
Figure:
Note: Adapted from Peng et al. A: PBMC responses in severe and mild cases when stimulated with viral peptides. B: Number of viral peptides recognized in severe and mild cases. Colored stacked bars represent different viral peptides. Permission request in process. Reprinted by permission from Springer Nature Customer Service Centre GmbH: Springer Nature, Nat Immunol. Peng et al. Broad and strong memory CD4+ and CD8+ T cells induced by SARS-CoV-2 in UK convalescent individuals following COVID-19. https://doi.org/10.1038/s41590-020-0782-6external icon, COPYRIGHT 2020.
- Consiglio et al. The immunology of multisystem inflammatory syndrome in children with COVID-19.external icon Cell. Description and comparison of the immune response for MIS-C versus Kawasaki disease, including differences for biomarkers to aid in diagnosis.
- Lee et al. Clinical significance of timing of intubation in critically ill patients with COVID-19: A multi-center retrospective study.external icon Journal of Clinical Medicine. A multicenter retrospective study of intubation timing in 47 patients with COVID-19 showed early intubation was not associated with improving survival.
- Kirenga et al. Characteristics and outcomes of admitted patients infected with SARS-CoV-2 in Uganda.external icon BMJ Open Respiratory Research. Describes COVID-19 in 56 patients at two hospitals in Uganda. COVID-19 cases were young and there was a high proportion of persons who were asymptomatic and had mild disease.
- Shuren et al. COVID-19 molecular diagnostic testing — Lessons learned.external icon NEJM. Perspective from two senior FDA officials that describes the Emergency Use Authorization (EUA) changes for SARS-CoV-2 testing and provides recommendations for future EUAs during pandemics.
- Wadman, M. Why COVID-19 Is More Deadly in People with Obesity—Even if they’re young.external icon Science. Reviews the increased risks of COVID-19 to individuals with obesity. The review describes potential causes for the increased risk of hospitalization.
- Guderian et al. In vitro comparison of surgical techniques in times of the SARS-CoV-2 pandemic: electrocautery generates more droplets and aerosol than laser surgery or drilling. external iconEuropean Archives of Oto-Rhino-Laryngology. Aerosol analysis of ENT procedures found that certain activities, such as drilling, laser ablation, and electrocauterization generated aerosols.
- Lachapelle, F. COVID-19 preprints and their publishing rate: An improved method (preprint)external icon. medRxiv. Only ~15% of COVID-19-related preprints went on to be peer-reviewed manuscripts between January and August 2020.
Disclaimer: The purpose of the CDC COVID-19 Science Update is to share public health articles with public health agencies and departments for informational and educational purposes. Materials listed in this Science Update are selected to provide awareness of relevant public health literature. A material’s inclusion and the material itself provided here in full or in part, does not necessarily represent the views of the U.S. Department of Health and Human Services or the CDC, nor does it necessarily imply endorsement of methods or findings. While much of the COVID-19 literature is open access or otherwise freely available, it is the responsibility of the third-party user to determine whether any intellectual property rights govern the use of materials in this Science Update prior to use or distribution. Findings are based on research available at the time of this publication and may be subject to change.