COVID-19 Science Update released: August 18, 2020 Edition 40

The COVID-19 Science Update summarizes new and emerging scientific data for public health professionals to meet the challenges of this fast-moving pandemic. Weekly, staff from the CDC COVID-19 Response and the CDC Library systematically review literature in the WHO COVID-19 databaseexternal icon, and select publications and preprints for public health priority topics in the CDC Science Agenda for COVID-19 and CDC COVID-19 Response Health Equity Strategy.
Here you can find all previous COVID-19 Science Updates.
Research on face coverings, including respirators (intended for use in occupational settings to protect the wearer) and commercially available or homemade masks (to be used as source control to protect others), is expanding. Material, fit, and other characteristics can affect face coverings’ ability to protect the wearer and/or others in their immediate vicinity. We present 3 recent studies that evaluate face coverings on these characteristics.
PEER-REVIEWED
Low-cost measurement of facemask efficacy for filtering expelled droplets during speechexternal icon. Fischer et al. Science Advances (August 7, 2020).
Key findings:
- Of all face coverings tested, the fitted (no valve) N95 was far superior to no mask; several other masks approached similar levels of performance (Figures 1 and 2).
- Poly/cotton and cotton pleated masks blocked over 80% of exhaled respiratory particles compared with no mask (Figure 2).
- Some face coverings, blocked substantially fewer (e.g., bandana) or effectively no (e.g. fleece gaiter) respiratory particles (Figure 2).
- Valved N95 offered some reduction of droplet spread; performance was likely affected by the valve.
Methods: Evaluation of the efficacy of 14 commonly available masks or mask alternatives to block respiratory particles droplets during regular speech. Each mask was compared to no mask on amount of droplet spread generated during speech. Limitations: Inter-subject variation due to mask fit, speech pattern; small study; limited measurement of droplet quantity and size.
Figure 1
Note: Adapted from Fischer et al. All masks used in study; 1) surgical; 2) valved N95; 3) knitted; 4) PolyProp (polypropylene); 5) Poly/Cotton (cotton-polypropylene-cotton); 6) MaxAT; 7) Cotton2; 8) Cotton4; 9) Cotton3; 10) Cotton1; 11) Gaiter-type neck fleece; 12) Double layer bandana; 13) Cotton5 (2-layer cotton, pleated style); 14) fitted (no valve) N95. Licensed under CC BY-NC 4.0.
Figure 2
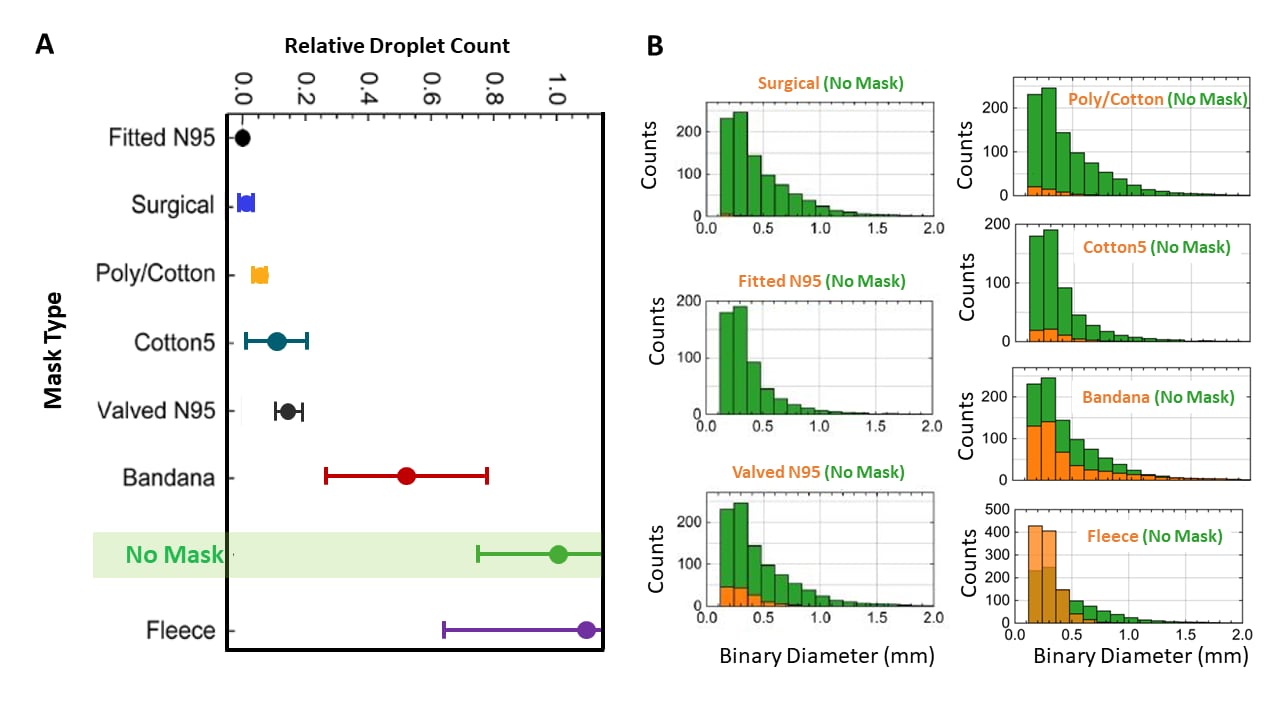
Note: Adapted from Fischer et al. A: Relative droplet count for selected masks: fitted (no valve) N95 respirator, surgical mask, poly/cotton mask, cotton mask, valved N95, bandana, fleece gaiter, and no mask. Solid data points and bars represent mean and SD results for 1 speaker over 10 trials. B: Number (y-axis) and size (x-axis) of particles for each selected mask type (in orange) relative to no mask (in green). Binary diameter is a measurement of light scattered by the particles (not the diameter of particles) into the camera aperture. Licensed under CC BY-NC 4.0.
Filtration efficiency of hospital face mask alternatives available for use during the COVID-19 pandemicexternal icon. Sickbert-Bennett et al. JAMA Internal Medicine (August 11, 2020).
Key findings:
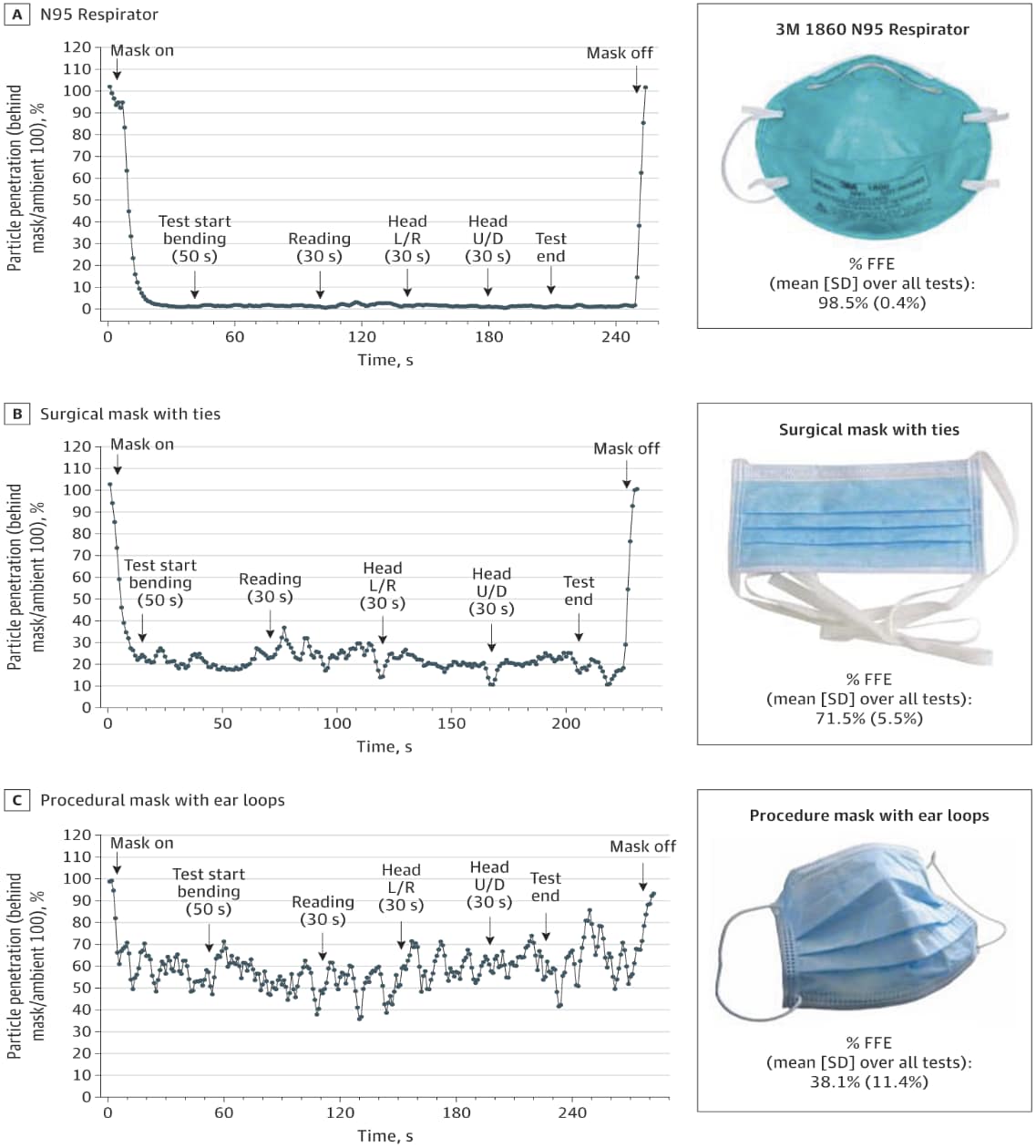
- Fitted filtration efficiency (FFE) for NIOSH-approved N95 respirators was 98.5% (Figure).
- Expired (<11 years) N95 respirators and those sterilized with ethylene oxide and vaporized hydrogen peroxide maintained FFEs >95%.
- FFE of surgical masks with ties (71.5%) and face masks with ear loops (38.1%) were substantially lower than N95 respirators (Figure).
- Most N95 respirators and surgical masks had comparable FFEs on a man and a woman, except for the surgical mask with ear loops (26.5% for the woman versus 39.5% for the man).
Methods: Measurement of FFE of 29 different respirators and face masks worn by a male and a female volunteer during a series of repeated movements of the torso, head, and facial muscles. Limitations: Testing was done on a one man (and one woman for some comparisons).
Figure:
Note: From Sickbert-Bennett et al. FFE with particle penetration through a facemask when worn with various activities for 30 or 50 seconds. L/R-left or right motion; U/D-up and down motion. A: NIOSH–approved N95 respirator; B: surgical mask with ties; C: mask with elastic ear loops. Reproduced with permission from JAMA Intern Med. doi:10.1001/jamainternmed.2020.4221. Copyright©2020 American Medical Association. All rights reserved
PREPRINTS (NOT PEER-REVIEWED)
Visualizing droplet dispersal for face shields and masks with exhalation valvesexternal icon. Verma et al. arXiv (July 31, 2020).
Key findings:
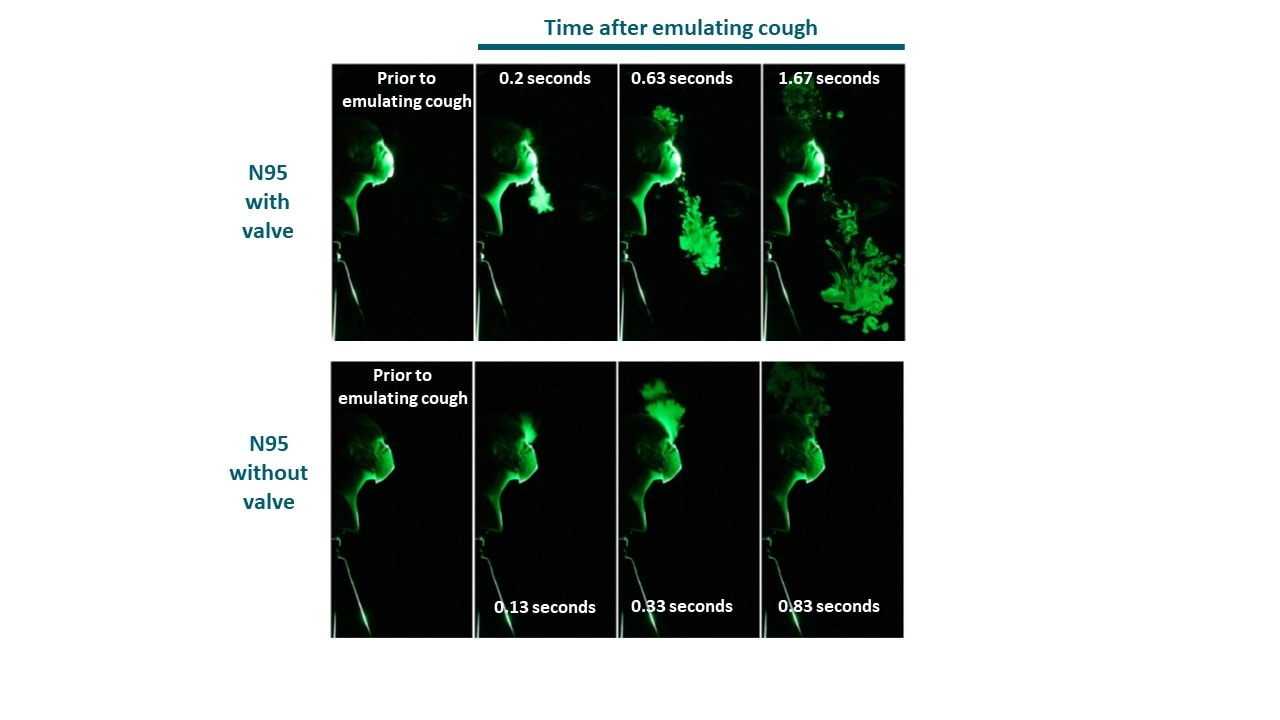
- An N95 respirator with an exhalation valve allowed most exhaled air to pass through the valve (Figure 1).
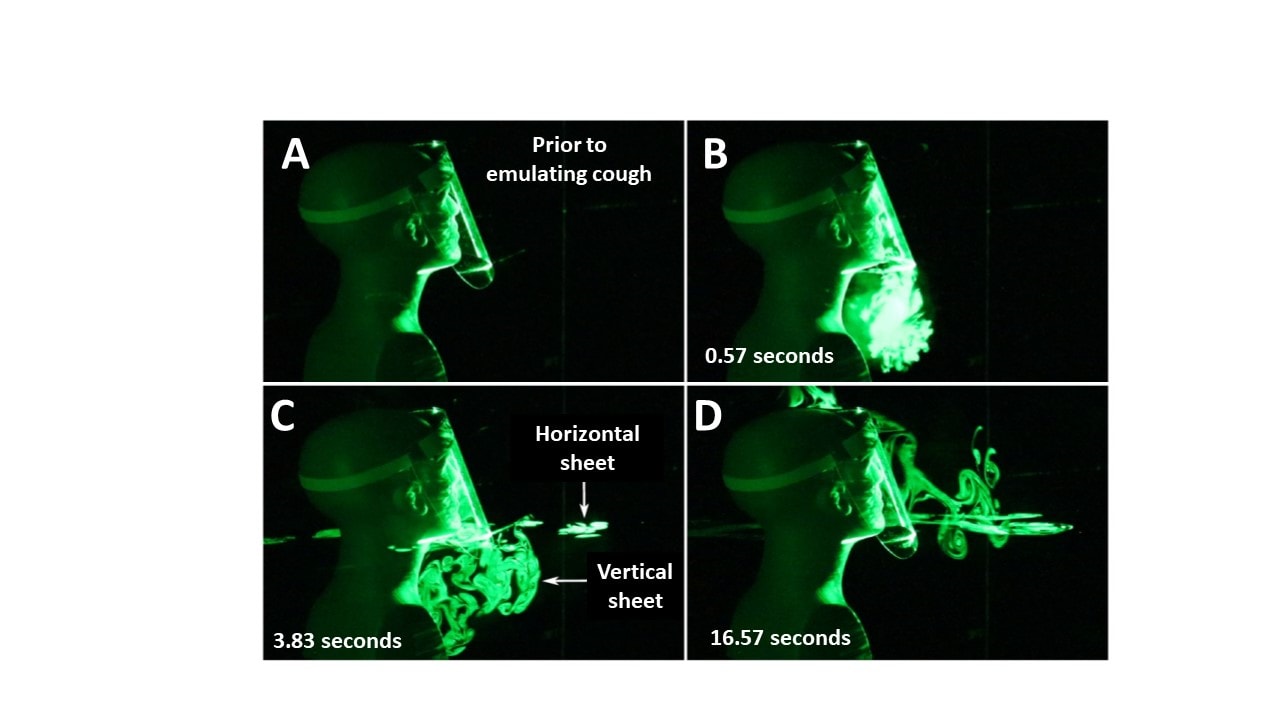
- A face shield initially deflected droplets downward, but with spread forward up and laterally after a few seconds (Figure 2).
- Commercially available surgical masks varied in amount of droplet leakage through the mask.
Methods: Performance of an N95 respirator with or without a valve, a face shield, or two surgical masks for effectiveness in reducing the spread of aerosol droplets. Coughs/sneezes were emulated with a manikin head wearing each device. Limitations: Qualitative visualization without standardized quantitation of particle counts or dispersion
Figure 1
Note: From Verma et al. Visualization of droplet spread when using an N95 respirator with an exhalation valve (top panels) and a N95 respirator without an exhalation valve (bottom panels). Used by permission from the author.
Figure 2
Note: From Verma et al. Droplet spread when a face shield is used. (A) Prior to emulating a cough/sneeze; (B) 0.57 seconds; (C) 3.83 seconds; (D) 16.57 seconds after the emulated cough. Used by permission from the author.
Implications for 3 studies (Fischer et al., Sickbert-Bennett et al., & Verma et al.): Purpose and performance need to be considered when choosing different face coverings. Face shields and masks with valves offer substantially less benefit than unvalved fabric masks and should not be used for this purpose.
PEER-REVIEWED
Racial/ethnic and socioeconomic disparities of SARS-CoV-2 infection among childrenexternal icon. Goyal et al. Pediatrics (August 5, 2020).
Key findings:
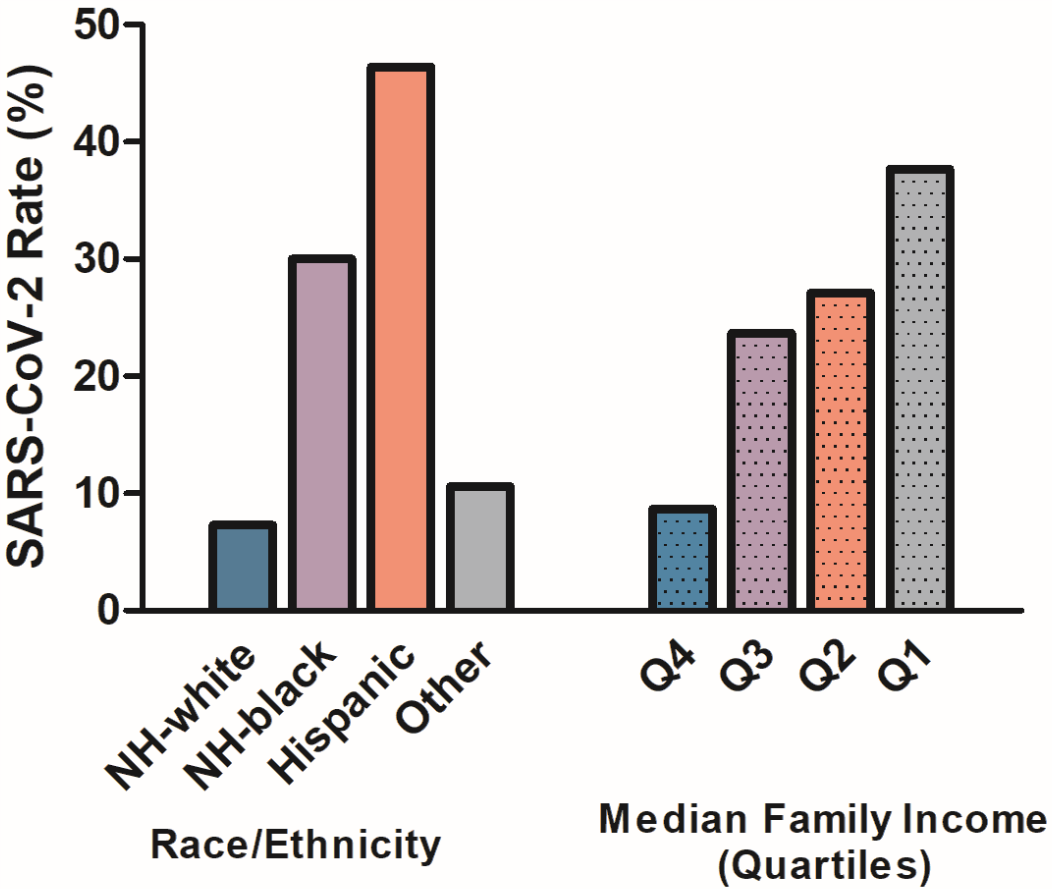
- Rates of SARS-CoV-2 infection were higher in non-Hispanic (NH) Black children (30.0%, OR 3.3 [95% CI 1.8-5.9]) and Hispanic children (46.4%, OR 9.1 [95% CI 5.1-16.4]) compared with NH White children (7.3%, referent), (Figure).
- Compared with SARS-CoV-2 infection rates among children in the highest family income quartile (8.7%, referent), infection rates were 23.7% (OR 3.2 [95% CI: 1.8-5.6]) in the 2nd income quartile, 27.1% (OR 3.8 [95% CI: 2.1-6.6]) in the 3rd quartile, and 37.7% (OR 5.9 [95% CI: 3.4-10.3]) in the lowest quartile (Figure).
Methods: Results of SARS-CoV-2 RT-PCR testing of NP or OP swabs from 1,000 persons aged < 1 year to 22 years March 21-April 28, 2020. Census block groups were used to estimate median family income. Limitations: Single testing site; non-random sample and denominator was children tested, based on symptoms and referral, rather than children in the population.
Implications: Racial/ethnic minority and socioeconomically disadvantaged children are disproportionately affected by SARS-CoV-2 infection. Modifiable factors driving these disparities should be addressed.
Figure:
Note: From Goyal et al. Rates of SARS-CoV-2 infection by race/ethnicity (solid-bars: non-Hispanic White, non-Hispanic Black, Hispanic, and other) and socioeconomic status (dotted bars: Quartile 4-highest income, Quartile 3, Quartile 2, Quartile 1-lowest income). Reproduced with permission from Pediatrics, e2020009951; DOI: https://doi.org/10.1542/peds.2020-009951external icon. Copyright © 2020 by the AAP.
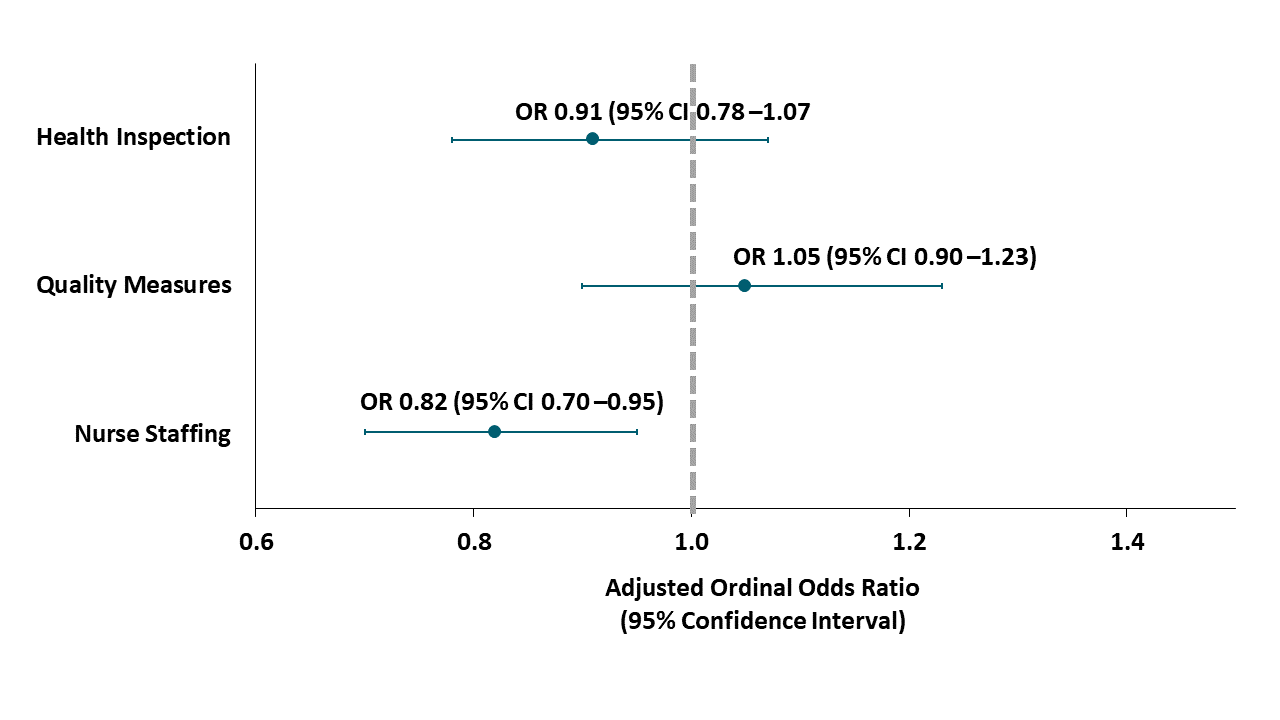
Association of nursing home ratings on health inspections, quality of care, and nurse staffing with COVID-19 cases.external icon Figueroa et al. JAMA (August 10, 2020).
Key findings:
- Among 4,254 nursing homes (NHs), 34.1% were ranked as “high performing” in terms of health inspections, 70.1% in terms of quality measures, and 35.9% in terms of nurse staffing.
- NHs that were ranked as high performing in terms of nursing staff were less likely to have > 30 cases of COVID-19 than NHs with 11-30 and <10 cases (Figure).
Methods: Cross-sectional study examining number of COVID-19 cases and NH performance as rated by CMS in terms of 3 domains (health inspection, quality measures, and nurse staffing) January 1 to June 30, 2020 in eight states. Odds that high-performing NHs compared with low-performing NHs had high number of COVID-19 cases (>30 cases vs. to 11-30 and <10 cases) according to each of these 3 domains were calculated and adjusted for certified number of beds in NHs. Limitations: No information provided regarding dates on which CMS ratings were obtained; limited to eight states; differential NH capacity to test and diagnose cases; no clinical outcomes.
Implications: NHs with staffing shortages might be vulnerable to COVID-19. Policies aimed at staffing support for NHs might help to mitigate disease spread.
Figure:
Note: Adapted from Figueroa et al. Ordinal odds of high-performing NHs having more than 30 COVID-19 cases (vs 11-30 cases and vs ≤10 cases) relative to low-performing facilities. Reproduced with permission from JAMA.doi:10.1001/jama.2020.14709. Copyright©2020 American Medical Association. All rights reserved
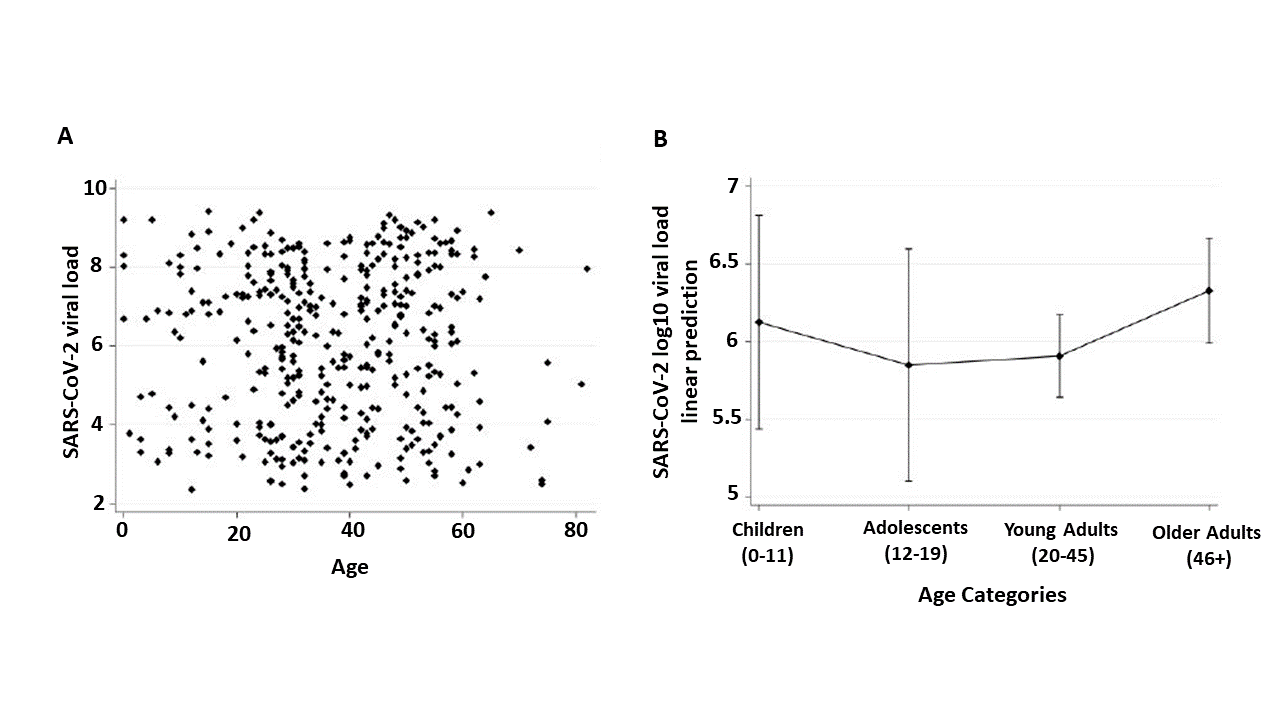
SARS-CoV-2 viral load in the upper respiratory tract of children and adults with early acute COVID-19external icon. Baggio et al. Clinical Infectious Diseases (August 6, 2020).
Key findings:
- Children, adolescents, young adults and older adults had similar viral load in NP swabs with no correlation observed between age and virus concentrations from NP swabs (Figure).
Methods: Cross-sectional study of viral load (range 2.4-9.4 Log10 RNA copies/mL) in NP swabs within 5 days (mean 2.3) after symptom onset in 53 children <16 years old and 352 adults ≥16 years old, March 10 to May 26, 2020, Geneva, Switzerland. Limitations: Symptomatic patients only; single center; small number of younger children.
Implications: Similar levels of virus in nasal passages of children and adults may indicate that symptomatic children may be as infectious as symptomatic adults. These findings are in contrast to those in Heald-Sargent et al.external icon described in the August 11, 2020 Science Update. Differences between these two studies could be due to study population, viral RNA quantification techniques, or analysis.
Figure:
Note: Adapted from Baggio et al. NP swab viral RNA Log10 copies per mL by age. A: Scatterplot of individual viral load values by age; B: Means and 95% CI of Log10 viral load by age group. Available via Oxford University Press Public Health Emergency Collection through PubMed Central.
PREPRINTS (NOT PEER-REVIEWED)
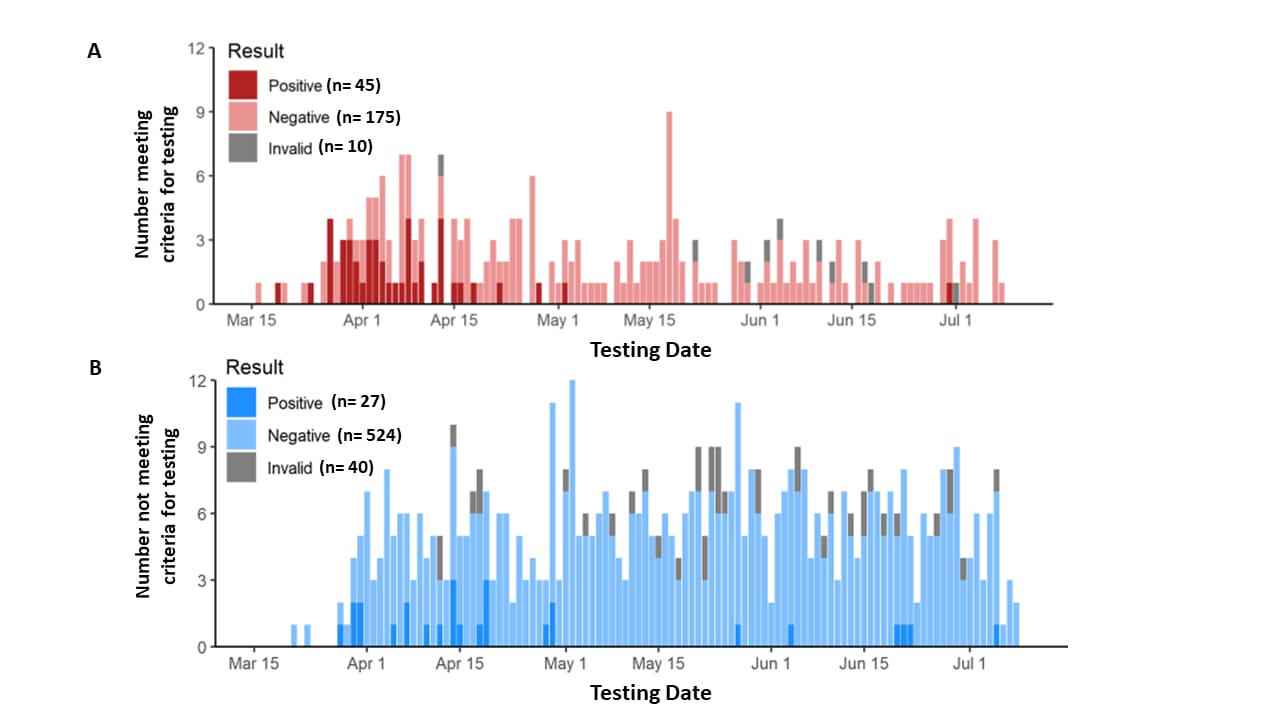
SARS-CoV-2 surveillance in decedents in a large, urban medical examiner’s officeexternal icon. Brouwer et al. medRxiv (August 3, 2020).
Key findings:
- 72/821 (8.8%) decedents assessed by medical examiner were SARS-CoV-2 positive, matching the general population prevalence.
- Decedents suspected of having had COVID-19 were more often positive than decedents randomly selected for SARS-CoV-2 testing (20% vs. 5%; p <0.001). (Figure).
- Deceased persons positive for SARS-CoV-2 RNA were more likely to be older and Black (all p-values <0.001).
- 55% of the tested decedents and 89% of those with a SARS-CoV-2 positive test were Black.
Methods: SARS-CoV-2 testing by NP RT-PCR performed for 230 decedents tested due to meeting criteria for suspected COVID-19 and 591 random decedents, March 16 to July 10, 2020, Michigan. Limitations: No serology; race but not ethnicity included; non-representative population sample.
Implications: Surveillance for SAR-CoV-2 among deceased persons may be useful. The large racial disparity in decedents with a positive test for SARS-CoV-2 underscores the disproportionate effect of COVID-19 among Black people.
Figure:
Note: From Brouwer et al. Daily SARS-CoV-2 test results among decedents who A: met criteria for COVID-19 testing; B: did not meet criteria for COVID-19 testing (randomly selected). Used by permission from the author.
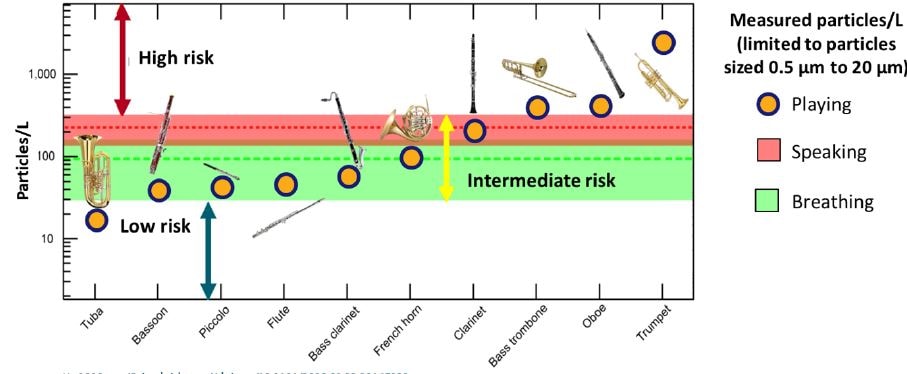
Aerosol generation from different wind instrumentsexternal icon. He et al. medRxiv (August 11, 2020).
Key findings:
- Production of respiratory particles from playing different brass and woodwinds exhibited wide variation (Figure).
- Compared with normal breathing and speaking, the tuba produced a lower concentration of respiratory particles in the 5-20 μm range, whereas other instruments produced intermediate (equivalent) or higher concentrations.
Methods: Concentration of respiratory particles 0.5-20 μm was measured when 10 different instruments were played and compared with that from normal speaking and breathing. Limitations: Instruments played alone and not in groups.
Implications: Understanding the concentrations of respiratory particles produced by different wind instruments can inform considerations for return-to-play decisions for various musical activities.
Figure:
Note: Adapted from He et al. Aerosol levels from 10 instruments. Average aerosol concentrations with breathing and speaking are shown by dashed horizontal lines and corresponding shaded regions represent the SD of these measurements. Used by permission from the author.
PREPRINTS (NOT PEER-REVIEWED)
Viable SARS-CoV-2 in the air of a hospital room with COVID-19 patientsexternal icon. Lednicky et al. medRxiv (August 4, 2020).
Key findings:
- Air samples collected 2.0-4.8 meters away from an infected patient were positive for SARS-CoV-2 by RT-PCR (Figure).
- When a high efficiency particulate air (HEPA) filter was attached to the sampling devices, no SARS-CoV-2 RNA was detected.
- Viral RNA concentrations from Patient 1 NP swab were higher than the four positive environmental samples (Ct of 24 versus 36-39, respectively).
- SARS-CoV-2 genomes sequenced from the positive air samples and Patient 1 matched.
Methods: Air samples were collected in a hospital room with two COVID-19 patients. At the time of air sampling Patient 1 was RT-PCR positive for SARS-CoV-2 and Patient 2 was RT-PCR negative by NP swab. Materials from air samples were tested with RT-PCR and cultured for SARS-CoV-2. Viral RNA from air samples and NP swab was sequenced. Limitations: Unclear if patients were symptomatic (coughing); no measure of air samples after patient 1 left the room.
Implications: Viable SARS-CoV-2 can be isolated from air up to 5 meters from infected patients; however, it is difficult to assess the epidemiological meaning of these results in the absence of data to assess whether what was detected in air samples could constitute an infectious dose.
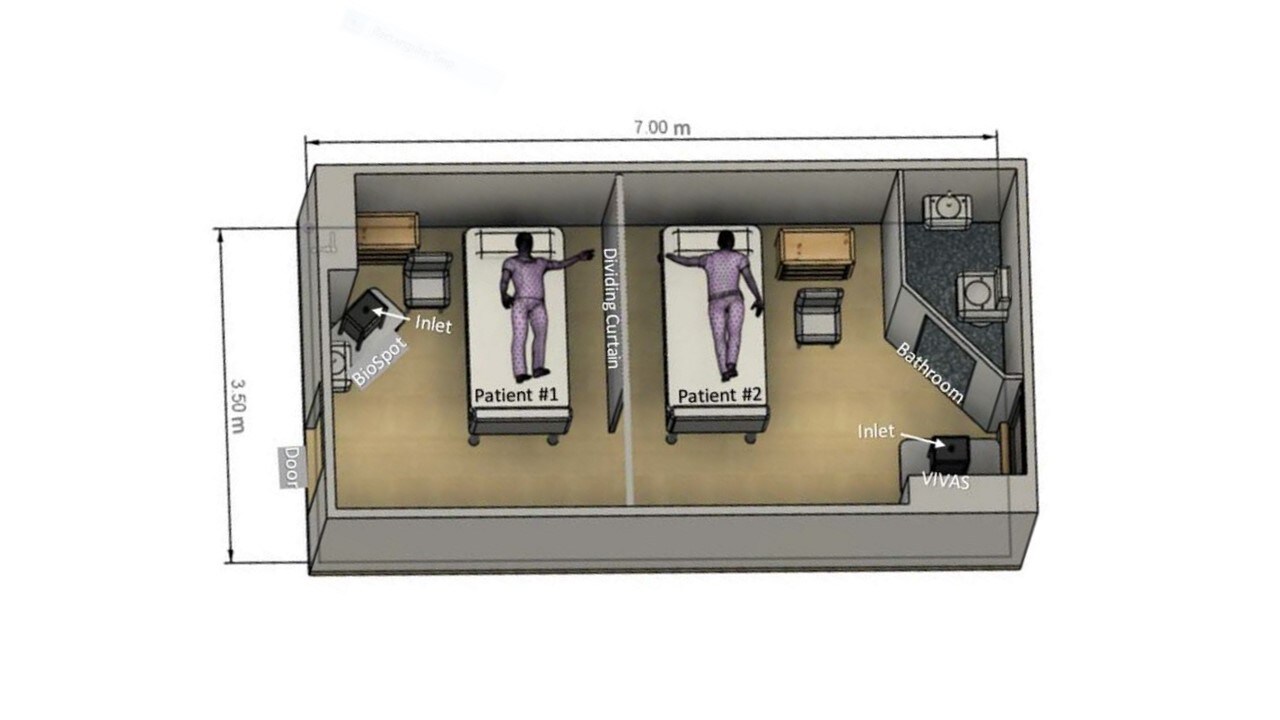
Figure:
Note: Adapted from Lednicky et al. Schematic diagram of room with patient bed and BioSpot and VIVAS air-sampler locations. Licensed under CC-BY-ND 4.0.
PEER-REVIEWED
Cardiac involvement in patients recovered from COVID-2019 identified using magnetic resonance imagingexternal icon. Huang et al. JACC: Cardiovascular Imaging (May 4, 2020).
Key findings:
- 15/26 (58%) patients had heart muscle (myocarditis) and lining (pericarditis) injury on magnetic resonance imaging (MRI).
- Compared with controls, recovered patients had decreased right ventricle function.
Methods: Retrospective study of 26 patients who recovered from COVID-19 pneumonia with no known prior heart disease, presenting with symptoms potentially related to cardiac disease (i.e., chest pain, palpitation, and chest distress) Wuhan, China, March to May, 2020. Participants underwent cardiac MRI 35 to 60 days after first reporting cardiac symptoms. Heart function was compared with 20 age- and sex-matched healthy controls. Limitations: MRI only performed on patients reporting symptoms; small sample; single center; short follow-up; adults only.
Implications: SARS-CoV-2 infection appears to cause at least short-term heart damage in a surprisingly large fraction of asymptomatic persons after resolution of the acute illness. Early detection and preventive strategies as well as longitudinal prospective clinical studies may be needed for patients recovering from COVID-19 to monitor and better understand the risk for transient or permanent cardiac injury.
More on Facemasks
- Teesing et al. Is there an adequate alternative for commercially manufactured face masks? A comparison of various materials and formsexternal icon. Journal of Hospital Infection. Masks made at home from commercially manufactured filter fabric, in duckbill form, perform better than many N95/FFP2/KN95 masks.
- Shack et al. Masked paediatricians during the COVID-19 pandemic and communication with childrenexternal icon. Journal of Paediatrics and Child Health. Pediatricians report more difficulty engaging with younger patients when mask-wearing and describe their ways of compensating.
International Findings
- Bielecki et al. Body temperature screening to identify SARS-CoV-2 infected young adult travellers is ineffectiveexternal icon. Travel Medicine and Infectious Disease. Body temperature among young adult men with COVID-19 is highly variable and likely ineffective for screening at international borders.
- Baker et al. Successful elimination of COVID-19 transmission in New Zealandexternal icon. NEJM. New Zealand’s approach to pandemic response included rapid, science-based risk assessment to inform government interventions at multiple levels.
- Mbow et al. COVID-19 in Africa: Dampening the storm? external icon Africa’s low rates of COVID-19 cases and deaths may be due in part to unreliable data, but also early mitigation actions, population characteristics such as age, and environmental exposures that strengthen immune response.
Other Topics
- Mahase E. COVID-19: Where are we on immunity and vaccines?external icon Describes current international vaccine development efforts and the need to expand research on T-cell immunity.
- Yu et al. Dysregulated adaptive immune response contributes to severe COVID-19external icon. Cell Research. Impaired cellular and enhanced humoral immune responses co-occur in COVID-19, suggesting that dysregulated adaptive immune responses may advance severe disease.
- Louisias et al. Intersectional identity and racial inequality during the COVID-19 pandemic: Perspectives of Black physician mothers. external iconJournal of Women’s Health. Offers strategies Black women physicians can use to cope with multiple sources of stress during the pandemic.
- Piller C. Data secrecy may cripple U.S. attempts to slow pandemic.external icon Researchers face ongoing challenges to accessing data on COVID-19 that could be useful to support response efforts.
Disclaimer: The purpose of the CDC COVID-19 Science Update is to share public health articles with public health agencies and departments for informational and educational purposes. Materials listed in this Science Update are selected to provide awareness of relevant public health literature. A material’s inclusion and the material itself provided here in full or in part, does not necessarily represent the views of the U.S. Department of Health and Human Services or the CDC, nor does it necessarily imply endorsement of methods or findings. While much of the COVID-19 literature is open access or otherwise freely available, it is the responsibility of the third-party user to determine whether any intellectual property rights govern the use of materials in this Science Update prior to use or distribution. Findings are based on research available at the time of this publication and may be subject to change.