COVID-19 Science Update released: August 6, 2021 Edition 101

The COVID-19 Science Update summarizes new and emerging scientific data for public health professionals to meet the challenges of this fast-moving pandemic. Weekly, staff from the CDC COVID-19 Response and the CDC Library systematically review literature in the WHO COVID-19 databaseexternal icon, and select publications and preprints for public health priority topics in the CDC Science Agenda for COVID-19 and CDC COVID-19 Response Health Equity Strategy.
Section headings in the COVID-19 Science Update align with the CDC Science Agenda for COVID-19.
Section headings in the COVID-19 Science Update have been changed to align
with the CDC Science Agenda for COVID-19.
Here you can find all previous COVID-19 Science Updates.
PEER-REVIEWED
Estimated mortality increases during the COVID-19 pandemic by socioeconomic status, race, and ethnicityexternal icon. Miller et al. Health Affairs (July 21, 2021).
Key findings:
- During the COVID-19 pandemic, higher all-cause mortality was observed in U.S. working-age adults without health insurance, with lower household income, with limited work-from-home options, living in adult correctional facilities, or living in health-related group quarters.
- Mortality rate was 1,619 for people living in health-related group quarters, 83 for people living in adult correctional facilities, and 16 for other adults.
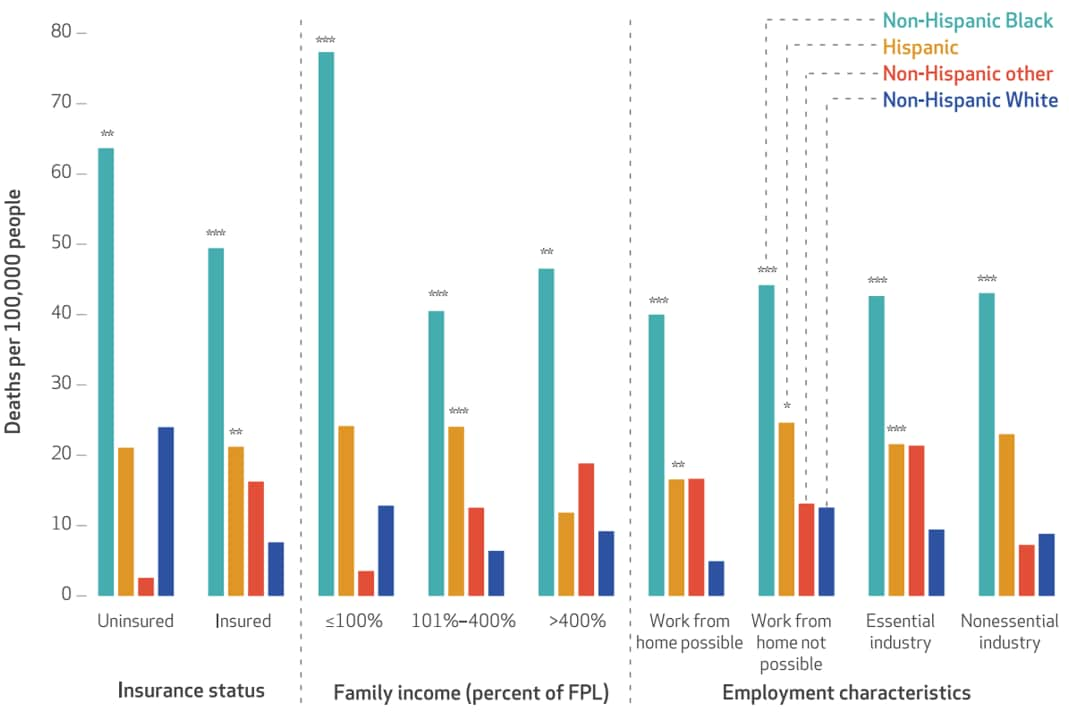
- Mortality rate varied significantly by race/ethnicity (Figure). Compared with non-Hispanic White adults:
- Mortality was higher among non-Hispanic Black adults, regardless of health insurance status, household income, or work-from-home options.
- Mortality was higher among Hispanic adults, among people with health insurance, not living in group quarters, lower income, with work-from-home options, and those in essential industries.
Methods: Data for adults (aged 19–64 years) were obtained from the American Community Survey (ACS) and correlated with mortality rate (per 100,000 population) reported by the Census Numerical Identification file. Limitations: Results do not indicate causation; mortality from COVID-19 not distinguished from other causes.
Implications: Race/ethnicity and socioeconomic characteristics including health insurance, household income, and employment characteristics may have impacted all-cause mortality in the COVID-19 pandemic.
Figure:
Note: Adapted from Miller et al. All-cause mortality from Q2 2019 to Q2 2020 in the United States based on health insurance status, household income, or employment characteristics and stratified by race/ethnicity (non-Hispanic Black, Hispanic, non-Hispanic other race/ethnicity, non-Hispanic White), *p<0.10, **p<0.05, ***p<0.01, Q = quarter, FPL = federal poverty level. Used by permission of publisher. Copyright 2021 by Project HOPE – The People-to-People Health Foundation, Inc.
PEER-REVIEWED
COVID-19 breakthrough infections in vaccinated health care workersexternal icon. Bergwerk et al. NEJM (July 28, 2021).
Key findings:
- Among 1,497 fully vaccinated healthcare workers in Israel who were tested for SARS-CoV-2 because of symptoms or known exposure over 14 weeks in early 2021, 39 (2.6%) had documented SARS-CoV-2 breakthrough infections. Most (85%) were B.1.1.7 (Alpha) variant, and 17 (59%) case-patients also had a concurrent positive rapid antigen test.
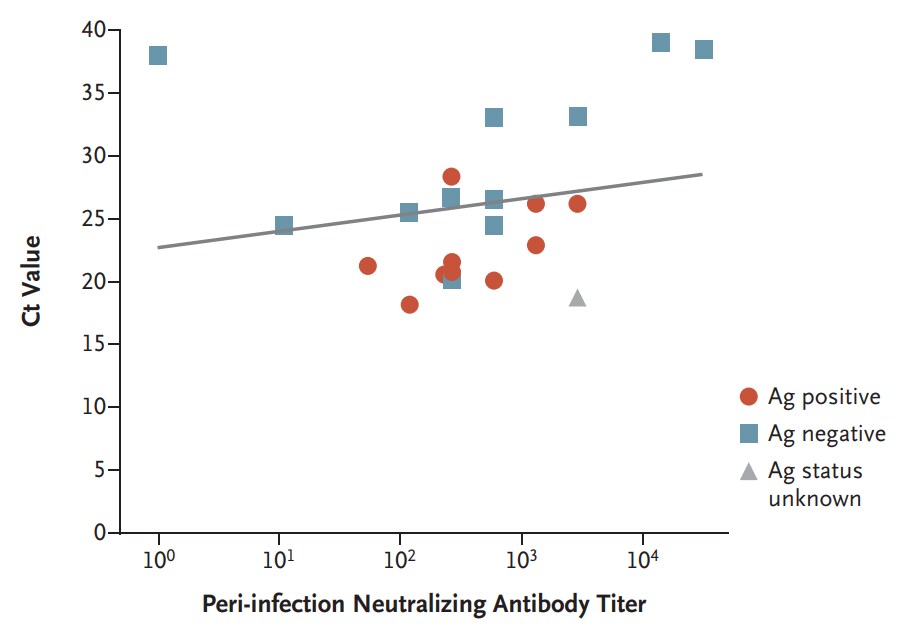
- 29 (74%) case-patients had a high viral load (Ct value <30) at some point during their infection (Figure), but no secondary infections were documented.
- 26 (67%) case-patients had mild infections, 13 (33%) were asymptomatic, and none required hospitalization, but 19% had symptoms persisting >6 weeks.
- Suspected source of infection was an unvaccinated person for 37 case-patients with available data: a household member for 21 (57%), and a patient or another healthcare worker for 11 (30%).
Methods: After 91% (n = 11,453) of healthcare workers at Israel’s largest medical center received 2 doses of BNT162b2 (Pfizer-BioNTech) vaccine from December 19, 2020 through April 28, 2021, breakthrough infections were identified among symptomatic or exposed healthcare workers from January 20, 2021 until April 28, 2021 using RT-PCR. Matched controls were used to identify correlates of infection, including asymptomatic infection. Limitations: Small number of cases; uncontrolled differences in exposure risk; asymptomatic cases could have been overlooked.
Implications: After 2 doses of BNT162b2 vaccine, few healthcare workers had breakthrough infections within 14 weeks; none required hospitalization, but some had persistent symptoms. Given high viral loads, potential onward transmission by vaccinated persons cannot be ruled out. However, no secondary infections were identified.
Figure:
Note: Adapted from Bergwerk et al. SARS-CoV-2 rapid antigen test status (Ag positive, Ag negative, or Ag status unknown) for 22 fully vaccinated healthcare workers with breakthrough infection for whom data were available, along with neutralizing antibody titers and N-gene cycle threshold (Ct) values (slope of regression line, 171.2; 95% CI 62.9-279.4). From the New England Journal of Medicine, Bergwerk et al., COVID-19 breakthrough infections in vaccinated health care workers. July 28, 2021, online ahead of print. Copyright © 2021 Massachusetts Medical Society. Reprinted with permission from Massachusetts Medical Society.
PREPRINTS (NOT PEER-REVIEWED)
Risk of myocarditis from COVID-19 infection in people under age 20: a population-based analysis.external icon Singer et al. medRxiv (July 27, 2021).
Key findings:
- Among 12- to 17-year-olds, 0.09% (6/6,846) of males and 0.04% (3/7,361) of females were diagnosed with myocarditis following SARS-CoV-2 infection.
- Among males, adjusted rate of myocarditis following COVID-19 was 450 cases per million, nearly 6 times higher than following vaccination.
- Among females, adjusted rate of myocarditis following COVID-19 was 213 cases per million, nearly 21 times higher than following vaccination.
Methods: Patient data for myocarditis cases (outcome diagnosis within 90 days) from 48 U.S. healthcare organizations (April 2020–March 2021) were acquired from TriNetX Research Network. Case numbers were adjusted based on cases reported outside TriNetX and COVID-19 case prevalence at the time. Limitations: Direct causation cannot be established; unreported cases would affect true prevalence of myocarditis.
Implications: Myocarditis is a rare outcome among youth aged 12–17 years that appears to be much more common following SARS-CoV-2 infection than following mRNA vaccination.
PEER-REVIEWED
Safety evaluation of the second dose of messenger RNA COVID-19 vaccines in patients with immediate reactions to the first dose.external icon Krantz et al. JAMA Internal Medicine (July 26, 2021).
Key findings:
- Study included 189 patients who experienced immediate allergic reactions after 1st mRNA COVID-19 vaccine dose (69% mRNA-1273, 31% BNT162b2).
- 17% (n = 32) met criteria for anaphylaxis.
- Subsequently, 159 (84%) patients received a 2nd mRNA COVID-19 dose, 47 (30%) with antihistamine premedication.
- 159 (100%) tolerated administration of the 2nd dose, including 19 who had experienced anaphylaxis previously.
- Most (80%) experienced no immediate symptoms after 2nd dose administration. 32 (20%) reported symptoms that were mild, self-limited, and/or resolved with antihistamines alone.
Methods: Multicenter, retrospective study among patients experiencing immediate allergic reaction to 1st dose of mRNA-1273 (Moderna) or BNT162b2 (Pfizer/BioNTech) from January 1 to March 31, 2021. Limitations: Study institutions did not share same evaluation protocol.
Implications: A 2nd dose of mRNA COVID-19 vaccine can be tolerated even by patients who experienced immediate allergic symptoms following their 1st dose.
PREPRINTS (NOT PEER-REVIEWED)
Cash versus lotteries: COVID-19 vaccine incentives experimentexternal icon. Duch et al. medRxiv (July 28, 2021).
Key findings:
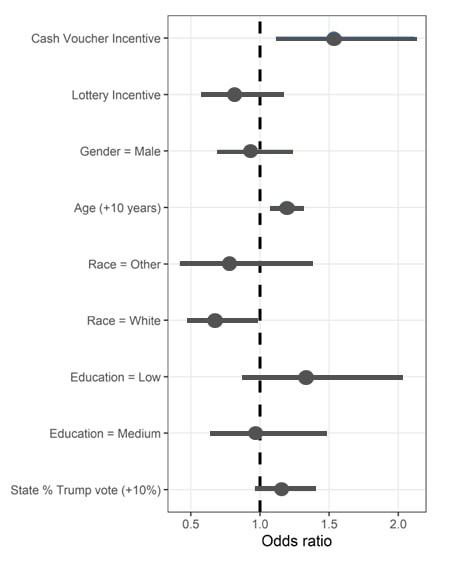
- Interest in receiving COVID-19 vaccination was significantly higher among participants randomized to watch a video mentioning cash incentives vs. health information alone (22% vs. 16%; adjusted OR [aOR] 1.5, 95% CI 1.1-2.1) (Figure).
- However, interest in vaccination was lower among participants randomized to watch a video mentioning lottery incentives vs. health information alone (14% vs. 16%; aOR: 0.8, 95% CI 0.6-1.2)
- Outcomes did not seem to differ by U.S. state partisanship.
Methods: Experimental study conducted June 28, 2021 through July 11, 2021 of 1,628 unvaccinated U.S. adults recruited online and randomly assigned to one of three 45-second interventions: a health information video (control); lottery drawing video; or up to $100 cash equivalent voucher video. Treatment effects were measured using a Bayesian logistic regression model with click-through to vaccination information as the outcome of interest. Limitations: Actual receipt of COVID-19 vaccination not assessed; participants not nationally representative; income and employment not measured; no dollar sensitivity analyses undertaken.
Implications: Cash or cash equivalents, but not lotteries, might be associated with slightly higher COVID-19 vaccination interest compared with health information alone, regardless of state partisanship.
Figure:
Note: Adapted from Duch et al. Odds ratios for interest in receiving COVID-19 vaccination. Licensed under CC-BY-NC-ND 4.0.
Tolerability, safety and immunogenicity of intradermal delivery of a fractional dose mRNA-1273 SARS-CoV-2 vaccine in healthy adults as a dose sparing strategyexternal icon. Roozen et al. SSRN (July 27, 2021).
Key findings:
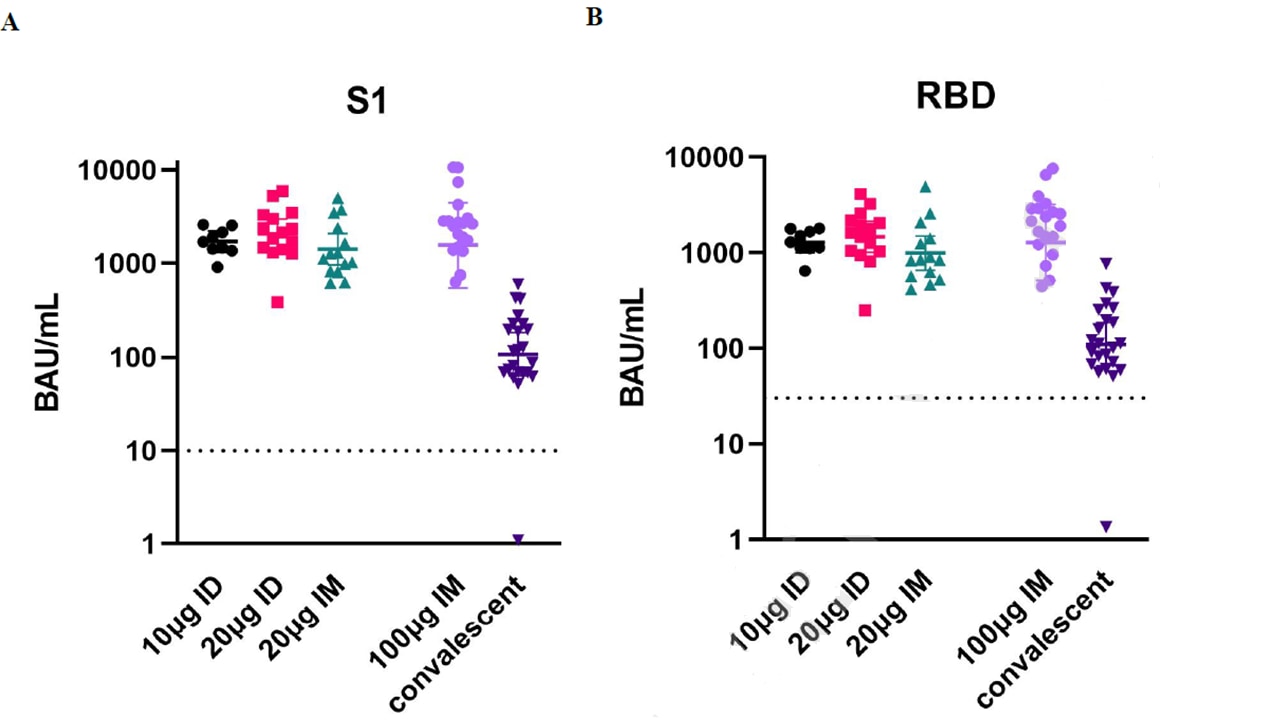
- 2 doses (28 days apart) 10 µg mRNA-1273 vaccine given intradermally (ID) elicited IgG anti-S1 and anti-RBD titers equivalent to 2 standard 100 µg doses given intramuscularly (IM) (Figure).
- 2 doses of 20 µg mRNA-1273 vaccine given either ID (n = 15) or IM (n = 14) also produced titers comparable to 2 standard 100 µg doses given IM. S1 and RBD-specific IgG concentrations were 14- to 20-fold higher than in convalescent sera from symptomatic patients.
- Self-reported adverse events were most commonly mild pain, itching, or erythema at the injection site.
Methods: Proof-of-concept, dose-escalation trial; participants received 2 doses of 10 µg mRNA-1273 (Moderna) given ID at a 28-day interval (n = 9), or 2 doses of 20 µg mRNA-1273 given ID (n = 15) or IM (n = 14). IgG-anti-S1 and IgG-anti-RBD antibodies were measured at day 43. Limitations: Small initial study; not assessed in persons aged >65 years; duration of immune response longevity not assessed beyond 43 days.
Implications: Similar immunogenicity, tolerability, and safety could be achieved using much smaller mRNA COVID-19 vaccine doses through intradermal rather than intramuscular administration, with potential dose-sparing implications.
Figure:
Note: Adapted from Roozen et al. Anti-SARS-CoV-2 antibody concentrations 43 days after 2-dose vaccination with 10 µg ID, 20 µg ID, 20 µg IM, or 2 doses 100 µg IM (reference group); convalescent samples were from symptomatic, non-hospitalized patients. (A) Anti-Spike S1; (B) Anti-receptor binding domain. Vaccinee median titers were significantly higher (p<0.0008) than convalescent sera. BAU = IgG concentrations in binding antibody units/mL. Symbols represent individual people. Values above dotted line are positive. Median titers in vaccinees were significantly higher (p<0.0008) than in convalescent sera. Permission request in process.
Prevention, Mitigation, and Intervention Strategies
- Thomas et al. Six month safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine.external icon medRxiv (Preprint; July 28, 2021). Published in NEJM as Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine through 6 monthsexternal icon (September 15, 2021). Among 42,094 participants (aged ≥12 years) in the ongoing placebo-controlled phase 2/3 efficacy trial of BNT162b2 (Pfizer/BioNTech) in 6 countries, through March 13, 2021, vaccine efficacy (VE) against laboratory-confirmed COVID-19 at 6 months post-2nd dose was 91.3% (95% CI 89.0%-93.2%) overall but varied by country. VE against severe disease was 96.7% (95% CI 80.3%-99.9%). In >12,000 participants with >6 months of safety follow-up, there were no deaths related to vaccination and no cases of myocarditis.
Note: Adapted from Thomas et al. Cumulative incidence of COVID-19 after 1st dose (Day 0) of either BNT162b2 or placebo. Filled symbols represent severe cases. The inset enlarges the initial 21-day period. Licensed under CC-BY-NC-ND 4.0.
- Ramanathan et al. Cell-mediated and humoral immune response to 2-dose SARS-CoV-2 mRNA vaccination in immunocompromised patient population.external icon medRxiv (Preprint; July 23, 2021). Following 2-dose mRNA vaccination (either BNT162b2 or mRNA-1273), among immunocompromised patients (n = 100), 69% had a positive qualitative result on interferon-γ release assay and 50% had a positive qualitative antibody result while 100% of controls (n = 16) had both. Absence of either cell-mediated or humoral immune response was most common in solid organ transplant recipients (38.1%) and patients with autoimmune disorders (35.3%).
- Stumpf et al. Humoral and cellular immunity to SARS-CoV-2 vaccination in renal transplant versus dialysis patients: A prospective, multicenter observational study using mRNA-1273 or BNT162b2 mRNA vaccine.external icon The Lancet Regional Health – Europe (July 23, 2021). In a prospective study of mRNA-vaccinated patients in Germany, vaccination-induced seroconversion 8 weeks after 1st dose was markedly lower in kidney transplant recipients (42% of 368) compared with dialysis patients (95% of 1,256) or medical personnel (98% of 144). Seroconversion induced by mRNA-1273 vs. BNT162b2 vaccines, respectively, was 97% vs. 88% in dialysis patients; 49% vs. 26% in kidney transplant patients; and 99% vs. 97% in medical personnel.
- Qin et al. Risk of breakthrough SARS-CoV-2 infections in adult transplant recipients.external icon Transplantation (July 23, 2021). Among 18,215 fully vaccinated solid organ transplant recipients at 17 transplant centers, 151 (0.8%) had breakthrough infections, 87 (57.6%) were hospitalized, and 14 (9.3%) died. Breakthrough infections and associated deaths were 82- and 485-fold more common among solid-organ transplant recipients than reported among fully vaccinated people in the general U.S. population through April 30, 2021.
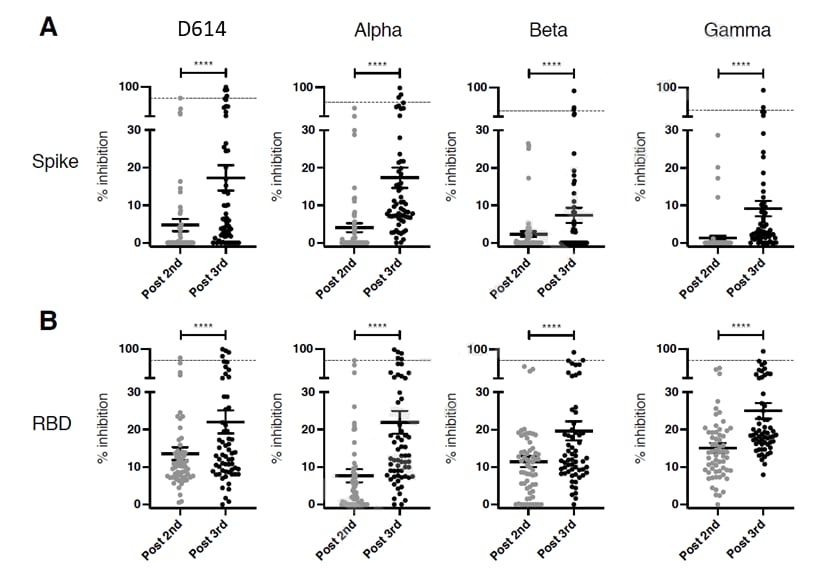
- Massa et al. Safety and cross-variant immunogenicity of a three-dose COVID-19 mRNA vaccine regimen in kidney transplant recipients.external icon SSRN (Preprint; July 22, 2021). Published in eBioMedicineexternal icon (November 8, 2021). In a prospective study in France, kidney transplant recipients who received a 3rd dose of BNT162b2 (n = 61) showed increases in both spike-specific antibody binding and in T-cell responses, although these remained lower than in healthy individuals. Neutralizing antibody responses against variants of concern also remained low.
Note: Adapted from Massa et al. Serum samples of kidney transplant recipients assayed for SARS-CoV-2 neutralizing antibodies using a pseudo-neutralization assay based on inhibition of binding of ACE2 receptor to (A) the SARS-CoV-2 spike, or (B) the receptor binding domain (RBD) of D614, B.1.1.7 (Alpha), B.1.351 (Beta), and P.1 (Gamma) variants. Dotted line shows mean percentage of inhibition measured in sera collected from healthy adults. Data are shown as mean and standard error of mean (SEM). ****p<0.0001 (paired comparisons). Permission request in process.
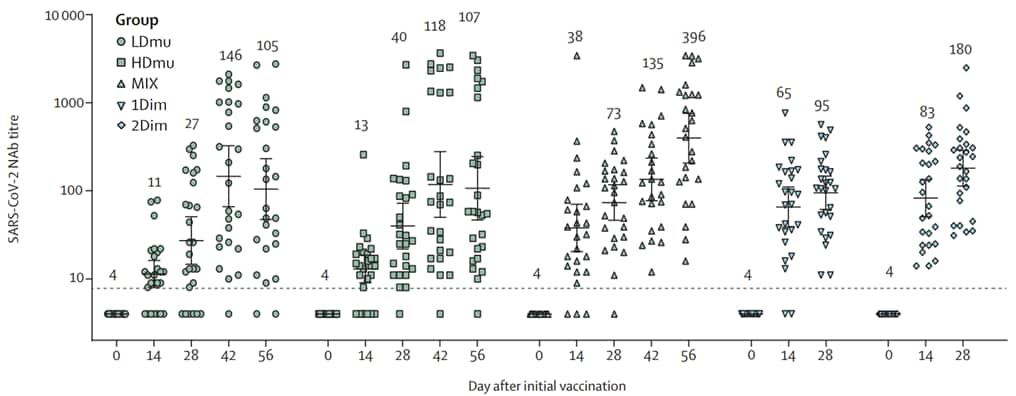
- Wu et al. Safety, tolerability, and immunogenicity of an aerosolised adenovirus type-5 vector-based COVID-19 vaccine (Ad5-nCoV) in adults: preliminary report of an open-label and randomised phase 1 clinical trial.external icon The Lancet Infectious Diseases (July 26, 2021). In a small phase 1 trial in China, 2 doses of an aerosolized form of Ad5-nCoV (CanSino Biologics) 28 days apart (1×10¹⁰ viral particles each dose) elicited similar neutralizing antibody titers to a single intramuscular dose of Ad5-nCoV (10×10¹⁰ viral particles). Titers were similar for one intramuscular dose followed by an aerosol booster. Adverse reactions were less common following mucosal aerosols than injections, and were mild among all participants.
Note: Adapted from Wu et al. SARS-CoV-2 neutralizing antibody following Ad5-nCoV vaccination. First dose given day 0 in all five study groups (n = 26 for each): LDmu (1×10¹⁰) and HDmu (2×10¹⁰) received aerosol followed at 28 days with 2nd doses of same potency; MIX received initial IM (5×10¹⁰) dose followed at 28 days by aerosol (2×10¹⁰). 1Dim received single IM dose (5×10¹⁰) and 2Dim received two IM doses. Geometric mean neutralizing titers at top. Reprinted from The Lancet Infectious Diseases, online July 26,2021, Wu et al., Safety, tolerability, and immunogenicity of an aerosolised adenovirus type-5 vector-based COVID-19 vaccine (Ad5-nCoV) in adults: preliminary report of an open-label and randomised phase 1 clinical trial, Copyright 2021, with permission from Elsevier.
Transmission of SARS-CoV-2
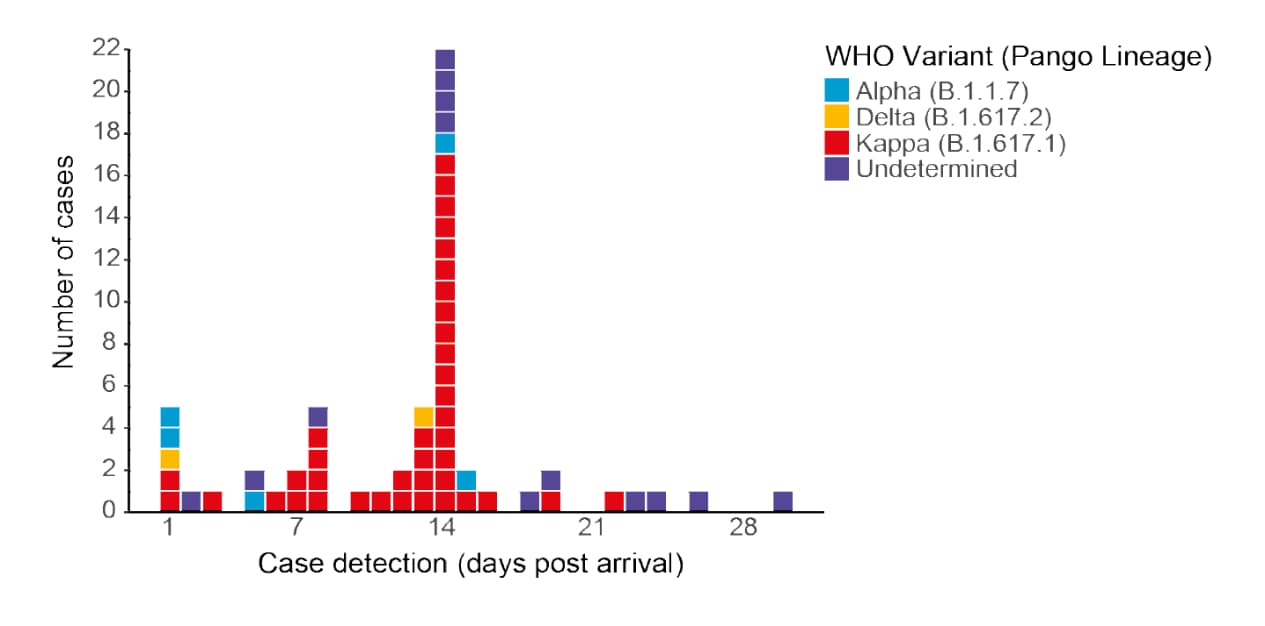
- Dhanasekaran et al. Air travel-related outbreak of multiple SARS-CoV-2 variants.external icon medRxiv (Preprint; July 23, 2021). Published in the Journal of Travel Medicineexternal icon (September 20, 2021). Despite a requirement that air travelers produce a negative nucleic acid test result for SARS-CoV-2 within 72 hours before departure from New Delhi, 7 of 146 passengers on an April 2021 flight tested positive on arrival in Hong Kong. During subsequent quarantine, an additional 52 cases were confirmed, ≥32 of which were Kappa, suggesting that infection occurred in flight or during quarantine, by a single source.
Note: Adapted from Dhanasekaran et al. A total of 59 cases of SARS-CoV-2, with colors indicating variant, were detected within 30 days post-arrival (day 1) of a 146-passenger flight. Licensed under CC-BY-NC-ND 4.0.
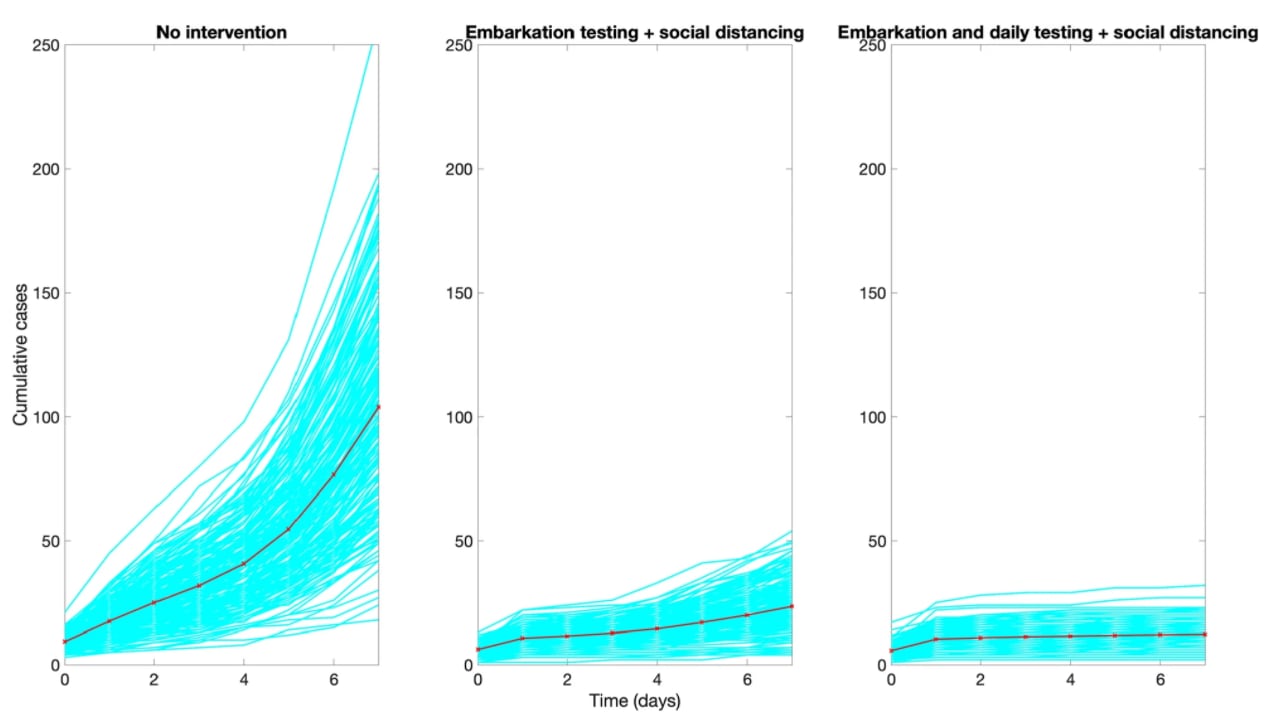
- Chowell et al. Harnessing testing strategies and public health measures to avert COVID-19 outbreaks during ocean cruises.external icon Scientific Reports (July 29, 2021). Mathematical modeling suggests that SARS-CoV-2 transmission aboard cruise ships could be slowed by adding daily on-board testing to pre-embarkation PCR testing, social distancing, and other public health measures.
Note: Adapted from Chowell et al. Modeling shows anticipated cumulative number of COVID-19 cases during a 7-day cruise with 465–2,000 crew members and 930–4,000 passengers, assuming no precautions (left); testing before departure and social distancing (middle); or testing before departure, daily testing, and social distancing (right). Red line indicates the mean of the 200 stochastic realizations. Licensed under CC BY 4.0.
Natural History of SARS-CoV-2 Infection
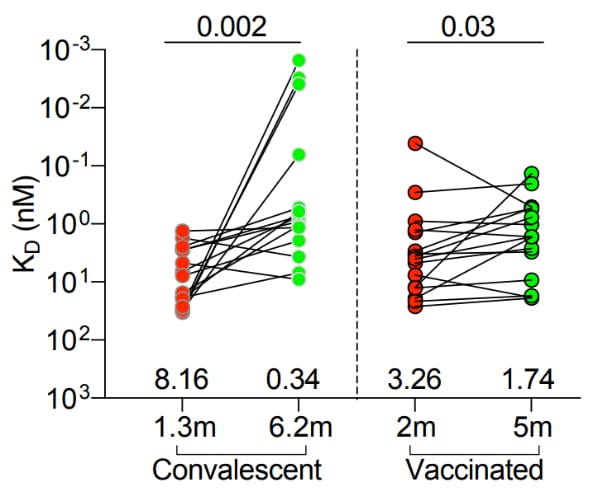
- Cho et al. Antibody evolution after SARS-CoV-2 mRNA vaccination.external icon bioRxiv (Preprint; July 29, 2021). Published in Nature as Anti-SARS-CoV-2 receptor-binding domain antibody evolution after mRNA vaccinationexternal icon (October 7, 2021). In the 5 months between 1st and 2nd doses of either mRNA vaccine, memory B-cells in SARS-CoV-2-naïve recipients increased neutralizing activity, but only to those SARS-CoV-2 strains that dominated the initial response. In contrast, B-cells produced in convalescents had greater potential breadth and potency against mutant pseudoviruses. This suggests that boosting naïve vaccinees with currently formulated mRNA vaccines might provide less protection against variants than vaccinating convalescents.
Note: Adapted from Cho et al. Antibodies to paired memory B-cell clones, shared across plasmablast, IgM and IgG prime, and IgG boost memory cells, isolated from convalescents 1.3 and 6 months after infection (n = 15) or individuals 2 and 5 months after full vaccination (n = 16). The lower the KD (equilibrium dissociation constant), the stronger binding affinity (y-axis). Numbers above x-axis indicate geometric mean values. Significance of change (p-values) at top. Licensed under CC-BY-NC 4.0.
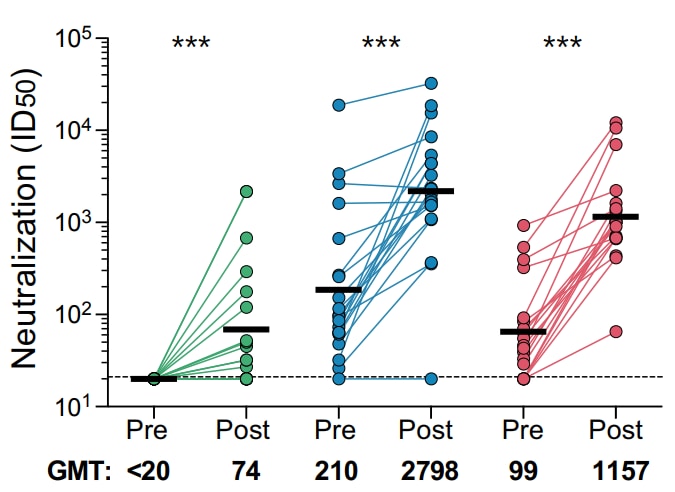
- Keeton et al. Prior infection with SARS-CoV-2 boosts and broadens Ad26.COV2.S immunogenicity in a variant dependent manner.external icon medRxiv (Preprint; July 28, 2021). Published in Cell Host & Microbeexternal icon (November 10, 2021). South African healthcare workers not previously infected with SARS-CoV-2 who received a single dose of Ad26.COV2.S (Janssen) vaccine produced significantly inferior neutralizing Ab against D614G than did those vaccinated after previous infection with ancestral D614G or B.1.351 (Beta). In contrast to antibody responses, generation of high-magnitude T-cell responses, including T-cell recognition of B.1.351, did not vary by prior infection.
Note: Adapted from Keeton et al. Neutralization of D614G pseudovirus by plasma pre- and post-vaccination with Ad26.COV2.S in participants with no prior infection (n = 19), those previously infected by D614G (n = 20), and those previously infected by B.1.351 ( n = 19). Neutralization is reflected as ID50 titer. Positivity threshold, dotted line. *** p<0.001. Licensed under CC-BY-NC-ND 4.0.
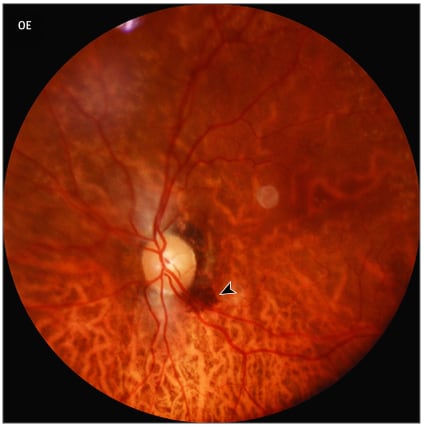
- Araujo-Silva et al. Presumed SARS-CoV-2 viral particles in the human retina of patients with COVID-19.external icon JAMA Ophthalmology (July 29, 2021). In 3 patients who died of COVID-19 in 2020, SARS-CoV-2 viral particles (presumed S and N proteins) were found in cells of the inner and outer nuclear layers of the retina, within endothelial cells close to the capillary flame, possibly explaining ocular manifestations of COVID-19.
Note: Adapted from Araujo-Silva et al. Fundus picture of patient 1’s left eye prior to death showing normal optic disc with sharp margins, a temporal subretinal hemorrhage (arrowhead), and increased vessel tortuosity. Reproduced with permission from JAMA Ophthamology, 2021. Published online July 29, 2021. https://doi.org/10.1001/jamaophthalmol.2021.2795. Copyright© 2021 American Medical Association. All rights reserved.
Social, Behavioral, and Communication Science
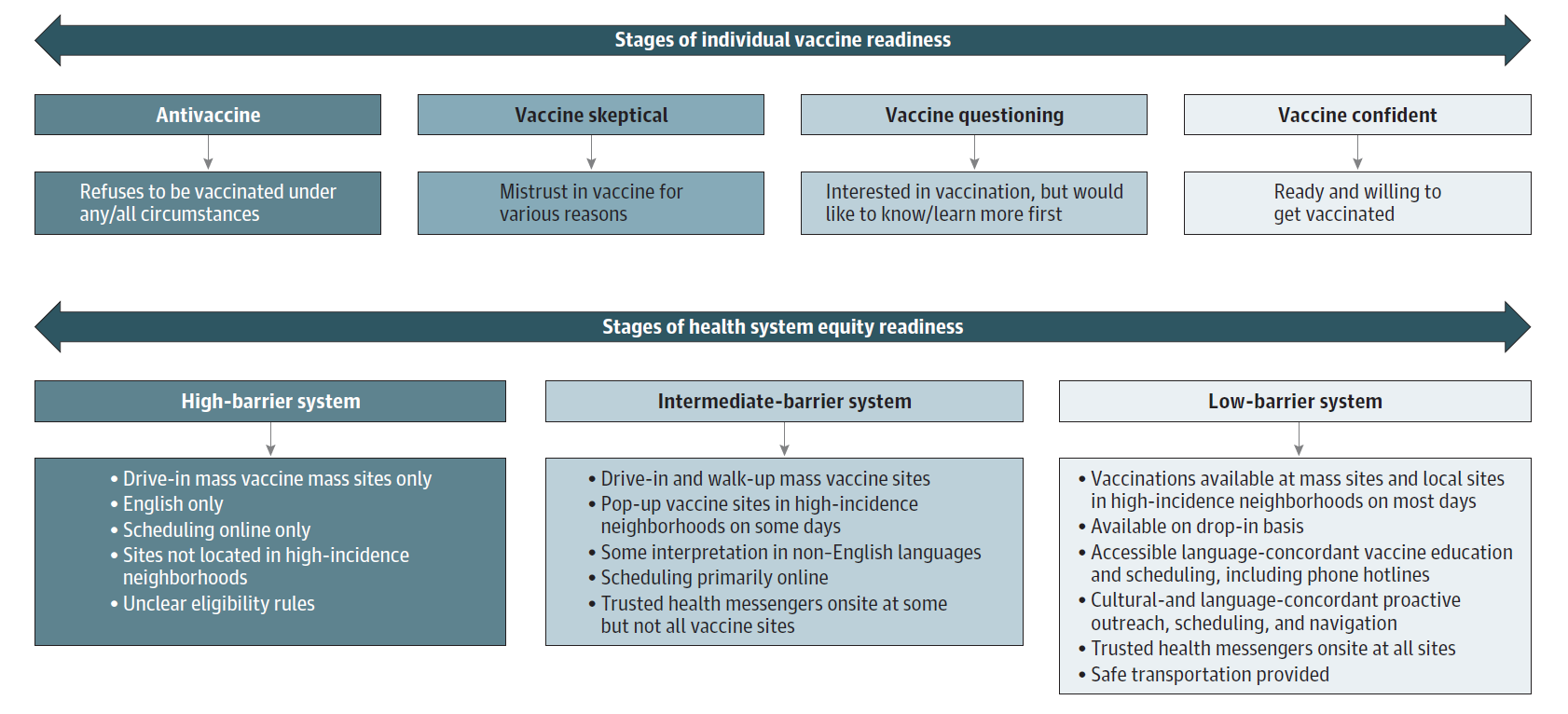
- Grumbach et al. Achieving racial and ethnic equity in COVID-19 vaccination: from individual readiness to health system readinessexternal icon. JAMA (July 30, 2021). Creating and sustaining low-barrier vaccination systems will be needed to achieve public health goals to increase community immunity in an equitable manner.
Note: Adapted from Grumbach et al. Stages of individual vaccine readiness: antivaccine, vaccine skeptical, vaccine questioning, vaccine confident. Stages of health system equity readiness: high-barrier system, intermediate barrier system, low barrier system.
Disclaimer: The purpose of the CDC COVID-19 Science Update is to share public health articles with public health agencies and departments for informational and educational purposes. Materials listed in this Science Update are selected to provide awareness of relevant public health literature. A material’s inclusion and the material itself provided here in full or in part, does not necessarily represent the views of the U.S. Department of Health and Human Services or the CDC, nor does it necessarily imply endorsement of methods or findings. While much of the COVID-19 literature is open access or otherwise freely available, it is the responsibility of the third-party user to determine whether any intellectual property rights govern the use of materials in this Science Update prior to use or distribution. Findings are based on research available at the time of this publication and may be subject to change.