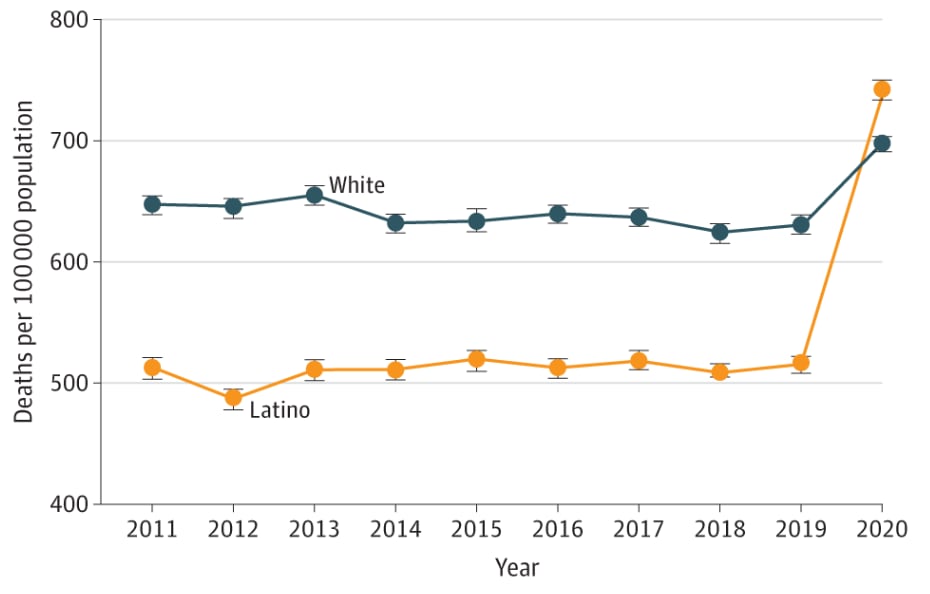COVID-19 Science Update released: July 30, 2021 Edition 100

The COVID-19 Science Update summarizes new and emerging scientific data for public health professionals to meet the challenges of this fast-moving pandemic. Weekly, staff from the CDC COVID-19 Response and the CDC Library systematically review literature in the WHO COVID-19 databaseexternal icon, and select publications and preprints for public health priority topics in the CDC Science Agenda for COVID-19 and CDC COVID-19 Response Health Equity Strategy.
Section headings in the COVID-19 Science Update align with the CDC Science Agenda for COVID-19.
Section headings in the COVID-19 Science Update have been changed to align
with the CDC Science Agenda for COVID-19.
Here you can find all previous COVID-19 Science Updates.
PEER-REVIEWED
Antibody response after a third dose of the mRNA-1273 SARS-CoV-2 vaccine in kidney transplant recipients with minimal serologic response to 2 doses.external icon Benotmane et al. JAMA (July 23, 2021).
Key findings:
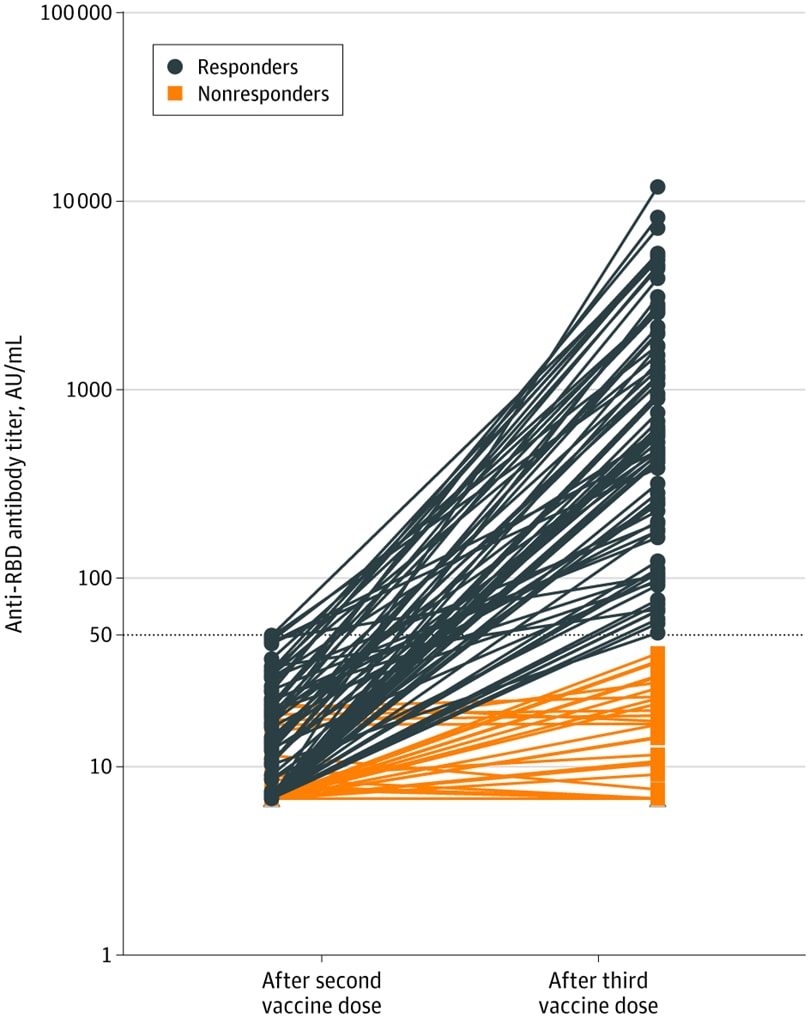
- Almost half (49%) of 159 kidney transplant recipients who did not respond (titer ≤49.9 AU/mL) after 2 doses of mRNA-1273 had an antibody response after a 3rd dose (median titer 586 AU/mL; IQR, 197.2-1920.1 AU/mL) (Figure).
- 35% of patients taking tacrolimus, mycophenolate, and steroids developed antibodies compared to 63% on other regimens.
Methods: Kidney transplant recipients (n = 159) with a negative COVID-19 history and SARS-CoV-2 antispike IgG <50 AU/mL on day of 1st vaccine dose and 1 month after 2nd dose were selected from a Kidney Transplantation Department in France between January 20, 2021 and June 3, 2021. A 3rd dose of mRNA-1273 (Moderna) was administered a median of 51 days following 2nd dose and antispike IgG response was measured 28 days later. Median time from transplantation was 5.3 years. Limitations: Lack of B- and T-cell studies.
Implications: Transplant patients on specific therapies may not be protected following 2 SARS-CoV-2 mRNA vaccine doses. These individuals should continue to follow current prevention measures (masking, distancing, avoiding crowds and poorly ventilated indoor spaces) after vaccination, and their close contacts should be vaccinated against COVID-19.
Figure:
Note: Adapted from Benotmane et al. Anti-receptor-binding domain IgG antibody titers measured 28 days after 3rd dose of mRNA-1273. Horizontal dotted line is cutoff for positivity. Blue lines are the antibody titers of kidney transplant recipients who seroconverted after 3rd dose (titers ≥50 AU/mL); orange lines, the evolution of antibody titers among nonresponders (titers <50 AU/mL). Reproduced with permission from JAMA. 2021; Online July 23, 2021. doi:10.1001/jama.2021. Copyright© 2021 American Medical Association. All rights reserved.
PREPRINTS (NOT PEER-REVIEWED)
Comparison of neutralizing antibody titers elicited by mRNA and adenoviral vector vaccine against SARS-CoV-2 variantsexternal icon. Tada et al. bioRxiv (July 21, 2021). Published in Frontiers in Immunology as Neutralization of SARS-CoV-2 variants by mRNA and adenoviral vector vaccine-elicited antibodiesexternal icon (March 8, 2022).
Key findings:
- Neutralizing antibody titers were consistently lower in individuals vaccinated with Ad26.COV2.S compared to natural infection, and vaccination with BNT162b2 or mRNA-1273.
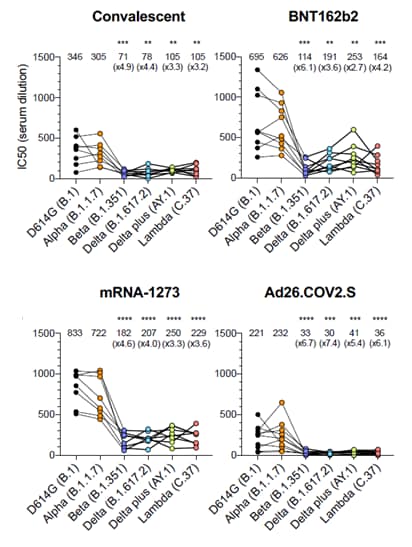
- Compared to D614G (B.1), loss of neutralization capacity for BNT162b2, mRNA-1273, and Ad26.COV2.S vaccinated sera against variants of concern were (Figure):
- 6.1-fold, 4.6-fold, and 6.7-fold less against Beta (B.1.351).
- 3.6-fold, 4.0-fold, and 7.4-fold less against Delta (B.1.617.2).
Methods: Serum samples were collected 32–57 days post symptom onset (convalescent, n = 8), ~ 90 days post 2nd dose for BNT162b2 (Pfizer/BioNtech, n = 10) and ~80 days post 2nd dose for mRNA-1273 (Moderna, n = 6). Ad26.COV2.S (Johnson & Johnson/Janssen) sera were collected ~82 days post single dose (n = 10). Limitations: Specimen number is small, so titer values should be interpreted with caution; unknown exactly how neutralizing antibody levels correlate with protection from infection.
Implications: Although Ad26.COV2.S showed reduced neutralizing antibody titers against Beta and Delta variants, it is unknown what titers correlate with protection from infection. Against moderate to severe-critical COVID-19, Ad26.COV2.S was previously foundexternal icon to perform well in a setting where Beta was the predominant circulating variant.
Figure:
Note: Adapted from Tada et al. Serum neutralizing antibody titers for D614G (wild-type), Alpha, Beta, Delta, Delta plus, and Lambda pseudovirus in convalescent individuals and BNT162b2, mRNA-1273, and Ad26.COV2.S vaccinated individuals. Top line of graphs shows IC50 titer for neutralization with fold decrease from D614G in parentheses. Lines in graphs are individual donors. Significant difference denoted by **, ***, ****, compared to D614G. Used by permission of author.
Effect of SARS-CoV-2 mRNA vaccination in MS patients treated with disease modifying therapies.external icon Sormani et al. SSRN (July 20, 2021). Published in eBioMedicineexternal icon (October 1, 2021).
Key findings:
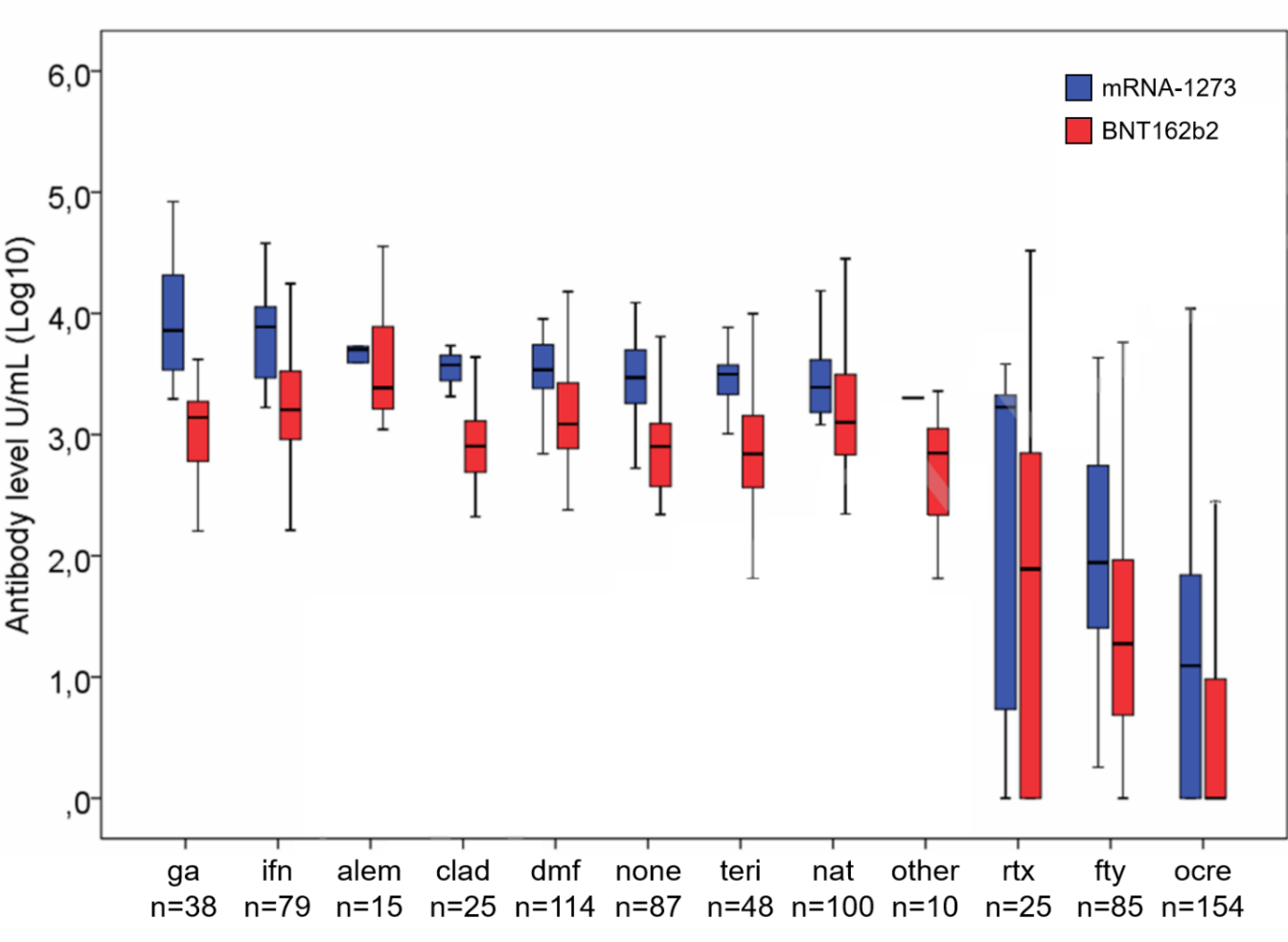
- Among fully vaccinated multiple sclerosis (MS) patients, neutralizing SARS-CoV-2 antibody responses were significantly reduced in patients treated with fingolimod (26-fold less, p <0.001) or with the anti-CD20 agents: rituximab (17-fold less, p <0.001) or ocrelizumab (178-fold less, p <0.001), compared to untreated patients.
- Vaccination with mRNA-1273 induced 3.5-fold higher antibody levels than BNT162b2.
Methods: MS patients in prospective multicenter cohort study in Italy were vaccinated with mRNA-1273 (Moderna, n = 186) or BNT162b2 (Pfizer/BioNTech, n = 594). Antibody responses to SARS-CoV-2 spike RBD were measured before 1st vaccine dose and 4 weeks after the 2nd vaccine dose. Limitations: Sample size small in some treatment groups.
Implications: MS patients treated with fingolimod, rituximab, or ocrelizumab had reduced immune response after mRNA vaccination.
Figure:
Note: Adapted from Sormani et al. SARS-CoV-2 spike RBD antibody levels in MS patients vaccinated with mRNA-1273 or BNT162b2 and separated by disease modifying treatment. ga = glatiramer-acetate, ifn = interferon, alem = alemtuzumab, clad = cladribine, dmf = dimethyl-fumarte, none = no MS therapy, teri = teriflunomide, rtx = rituximab, fty = fingolimod, ocre = ocrelizumab. Permission request in progress.
Immunogenicity of mRNA-1273, BNT162b2 and Ad26.COV2.S COVID-19 vaccinesexternal icon. Naranbhai et al. medRxiv (July 22, 2021). Published in Journal of Infectious Diseases as Comparative immunogenicity and effectiveness of mRNA-1273, BNT162b2, and Ad26.COV2.S COVID-19 vaccinesexternal icon (December 9, 2021).
Key findings:
- In adults earlier infected with SARS-CoV-2, 1 dose of mRNA-1273 (n = 5), BNT162b2 (n = 7) or Ad26.COV2.S (n = 14) were equally immunogenic.
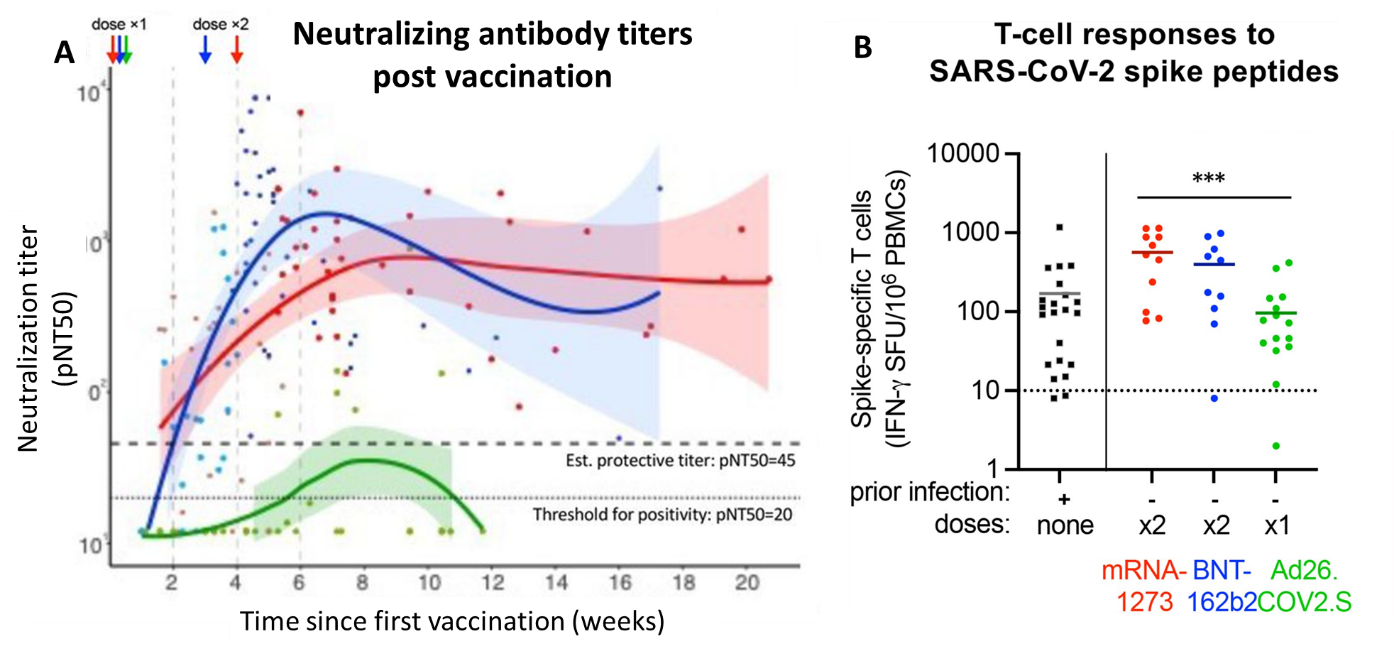
- In infection naïve adults, single dose Ad26.COV2.S (n = 22) induced significantly lower neutralizing antibody than did 1 dose of mRNA-1273 (n = 30) or BNT162b2 ( n = 21); the gap widened after 2 doses of mRNA (Figure A).
- At a median of 66 days (~ 9 weeks) post vaccination, 73% (11/15) of Ad26.COV2.S recipients remained negative for neutralizing antibody.
- Ad26.COV2.S induced fewer T cell responses (96 SFU) compared to mRNA-1273 (564 SFU) or BNT162b2 (398 SFU) (Figure B).
Methods: Immune response was measured in sera from healthy adults from Massachusetts vaccinated between February and March 2021 with either BNT162b2 (Pfizer/BioNTech, 1 dose n = 28, 2 doses n = 54), mRNA-1273 (Moderna, 1 dose n = 35, 2 doses n = 62), or Ad26.COV2.S (Johnson & Johnson/Janssen, 1 dose n = 36), stratified by previous SARS-CoV-2 infection . Viral neutralization was performed using the Wuhan isolate of SARS-CoV-2. Limitations: Small sample size.
Implications: A single dose Ad26.COV2.S appears to be less immunogenic than mRNA vaccination in people without prior SARS-CoV-2 infection; among individuals with prior infection, vaccination conferred higher antibody concentration and neutralization titers regardless of which vaccine was administered.
Figure:
Note: Adapted from Naranbhai et al. (A) Neutralization titers following vaccination with mRNA-1273, BNT162b2, Ad26.COV2.S. Time of doses are shown by arrows at the top left of the figure. Data fit with Loess regression and 95% confidence intervals shown in shaded regions. (B) T-cell response: interferon γ spot forming units per 10e6 peripheral blood mononuclear cells are shown for naturally infected versus fully vaccinated individuals who received either mRNA-1273, BNT162b2, or Ad26.COV2.S. Significant difference denoted by ***. PBMC = peripheral blood mononuclear cells. Dashed line indicates positive threshold. Used by permission of author.
PEER-REVIEWED
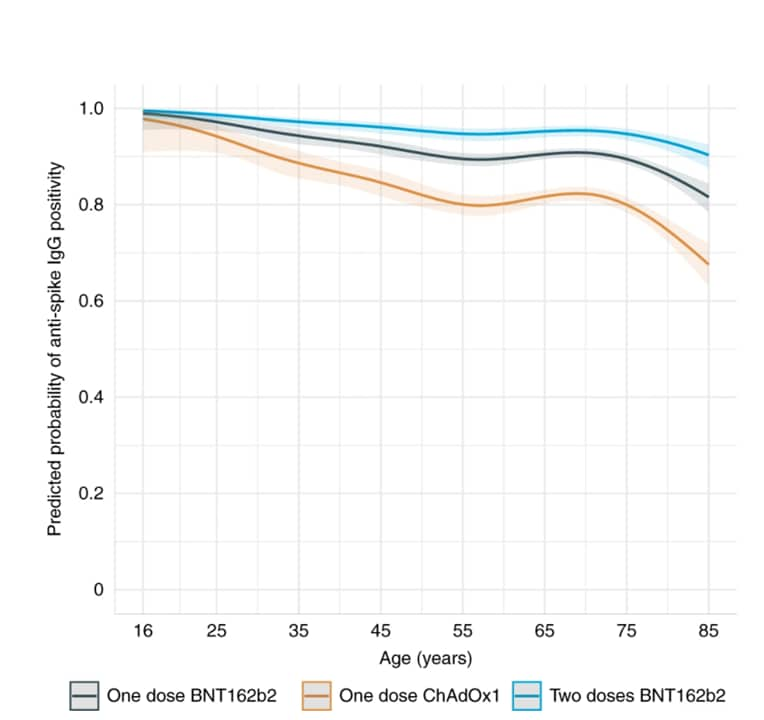
Antibody responses to SARS-CoV-2 vaccines in 45,965 adults from the general population of the United Kingdomexternal icon. Wei et al. Nature Microbiology (July 21, 2021).
Key findings:
- Following 1st dose of either vaccine, previously infected adults achieved higher antibody responses compared to those uninfected in all age groups.
- Among infection-naïve adults, antibody levels were lower after 1 dose of ChAdOx1 compared with 1 dose of BNT162b2 (Figure).
- 80-year-olds were less likely to be seropositive after a single dose of either vaccine vs. 40-year-olds:
- ChAdOx1: 74% (95% CI 66-80%) vs. 84% (95% CI 76-89%).
- BNT162b2: 85% (95% CI 80-89%) vs. 95% (95% CI 92-97%).
- Seropositivity was less likely among those with long-term health conditions (adjusted OR = 0.64 [0.60–0.69]) at >60 days after 1st dose of either vaccine.
- 80-year-olds were less likely to be seropositive after a single dose of either vaccine vs. 40-year-olds:
Methods: Representative sample of UK population ≥16 years of age (n = 45,965) who were first vaccinated between December 8, 2020 and April 6, 2021. SARS-CoV-2 anti-spike IgG was measured from 91 days before 1st vaccine dose until April 6, 2021. Probability of anti-spike IgG positivity after 1st vaccination was modeled by age and time. Limitations: Insufficient data to analyze participants with 2 doses of ChAdOx1; fewer data on younger adults due to vaccination prioritization of older adults; data gathered prior to dominance of B.1.617.2 SARS-CoV-2 in the UK.
Implications: Vaccination boosts antibody responses in individuals with previous SARS-CoV-2 infection and is likely to provide further protection against reinfection; when vaccine supplies are limited, 2nd doses could be prioritized for individuals without previous infection.
Figure:
Note: Adapted from Wei et al. Predicted probability of anti-spike IgG positivity 14–60 days after 1st vaccination in 24,977 participants without evidence of prior infection by age and vaccine type. Age was fitted using natural cubic spline with 4 internal knots placed at the 20th, 40th, 60th, and 80th percentile (30, 44, 57, and 71 years) and 2 boundary knots at the 5th and 95th percentile (19 and 82 years). The 95% CIs shown as the shaded area. Licensed under CC BY 4.0.
PEER-REVIEWED
Household transmission of SARS-CoV-2 from children and adolescents.external icon Chu et al. NEJM (July 21, 2021).
Key findings:
- Risk of SARS-CoV-2 transmission was decreased when the index case physically distanced (adjusted OR 0.4 95% CI 0.1-0.9) in the household.
- 79% of household transmission cases were from index cases who became symptomatic after returning home.
- Median time between index case symptoms and symptoms from household members emerging was 5 days.
Methods: Retrospective cohort study of household COVID-19 transmission from index patients (n = 224, 7–19 years old) who contracted COVID-19 from an overnight camp outbreak in June 2020. There were 526 household contacts. Limitations: COVID-19 test results were self-reported, testing was voluntary, and many cases had not yet returned home when they first became symptomatic, likely underestimating actual household transmission.
Implications: Physical distancing can minimize household COVID-19 transmission from children.
Detection, Burden, and Impact
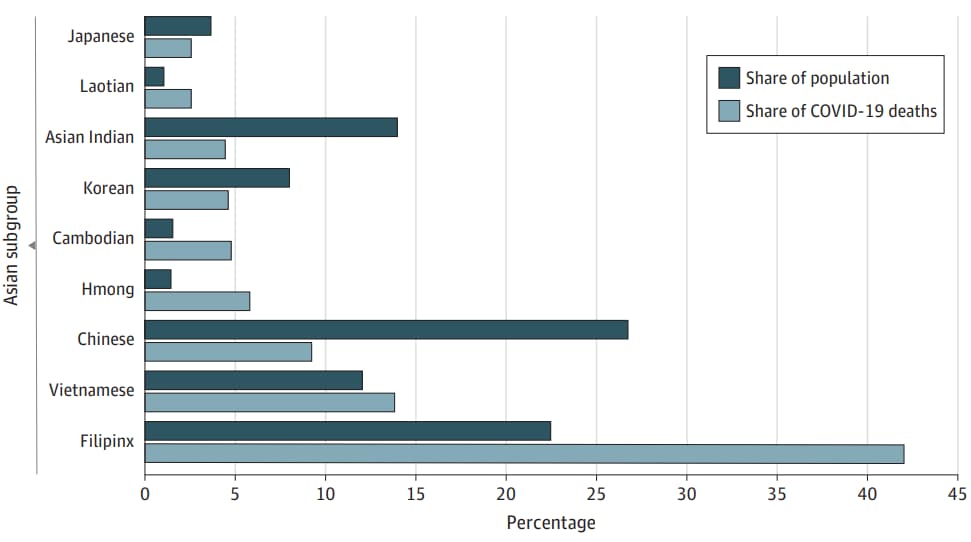
- Oronce et al. US health care relies on Filipinxs while ignoring their health needs: disguised disparities and the COVID-19 pandemic.external icon JAMA Health Forum (July 23, 2021). Of the 2.9 million Filipinxs living in the U.S., 38% of households are multigenerational and include at least 1 healthcare worker, twice the proportion for other Asian Americans. Despite constituting only 20% of California’s Asian population, they comprised 42% of COVID-19 deaths in 2020. This disproportionate burden is obscured by aggregating statistics for Americans of Asian descent.
Note: Adapted from Oronce et al. Filipinx share of COVID-19 deaths among Asians and share of the Asian population of California in 2020, by single race non-Latino Asian subgroup of adults (18–64 years). Licensed under CC-BY.
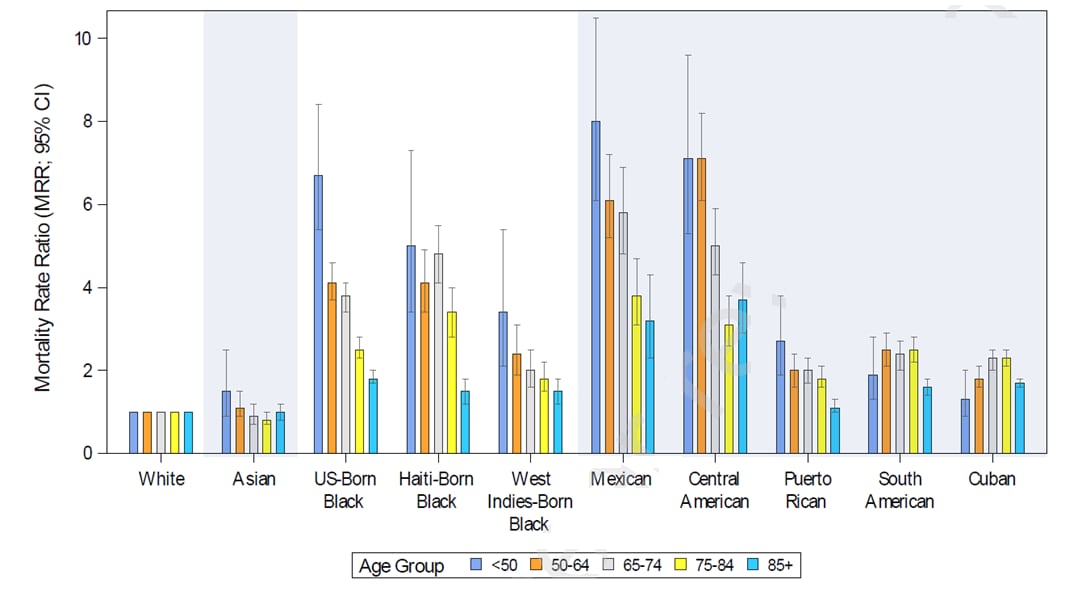
- Pinheiro et al. New insights into the burden of COVID-19 mortality for U.S. Hispanics and Blacks — when examined by country/region of origin. external iconSSRN (Preprint; July 20, 2021). Published in The Lancet Regional Health – Americas as New insights into the burden of COVID-19 mortality for U.S. Hispanics and Blacks when examined by country/region of origin: An observational studyexternal icon (November 5, 2021). Age-adjusted mortality ratios (AAMR, per 100,000) for Floridians in 2020 (n = 18,342) were lower for Whites (39.3) than for Hispanics (86.8) or Blacks (107.6). There were, however, differences among Black and Hispanic ethnic identities. Mexican and Central American decedents had the highest AAMR (170.7 and 168.8) and lowest age at death, but fewest comorbidities. Compared to young non-Hispanic Whites, Mexicans and Central Americans <50 years had much higher MRRs of 8.0 [95% CI 5.9-10.5] and 7.1 [95% CI 5.3-9.6].
Note: Adapted from Penheiro et al. Mortality rate ratios (MRRs) for 2020 Florida COVID-19 deaths by age group, race, ethnicity, and country/region of origin. Permission request in progress.
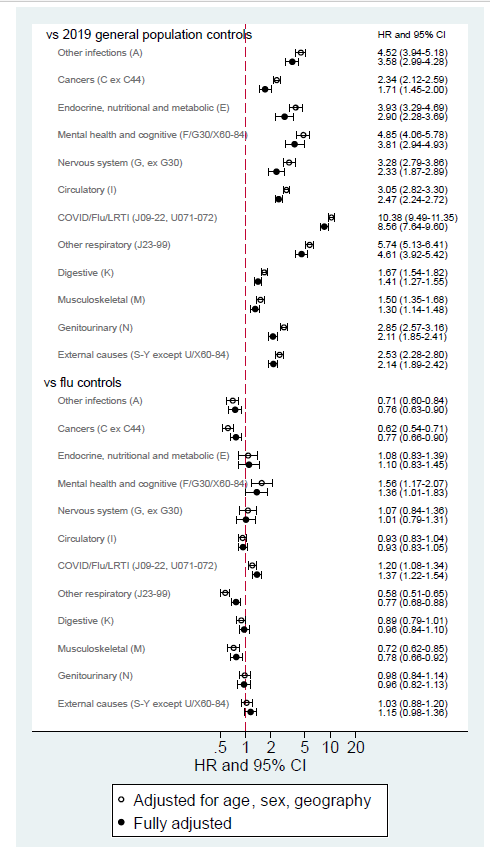
- Bhaskaran et al. Overall and cause-specific hospitalisation and death after COVID-19 hospitalisation in England: cohort study in OpenSAFELY using linked primary care, secondary care and death registration data.external icon medRxiv (Preprint; July 19, 2021). Published in PLOS Medicine as Overall and cause-specific hospitalisation and death after COVID-19 hospitalisation in England: A cohort study using linked primary care, secondary care, and death registration data in the OpenSAFELY platformexternal icon (January 25, 2022). Among 24,673 post-discharge COVID-19 patients in England followed for ≤315 days, overall risk of hospitalization or death (30,968 events) was similar to that of 16,058 influenza controls. They were, however, more likely than influenza patients to be readmitted or die due to lower respiratory tract infections (adjusted HR 1.37, 1.22-1.54) or cognitive-related illness (adjusted HR 1.36, 1.01-2.83).
Note: Adapted from Bhaskaran et al. Hazard ratios comparing prior COVID-19 hospitalization and controls for cause-specific hospital admission or death, adjusted for age, sex, and geography. Fully adjusted models also included ethnicity, obesity, smoking status, deprivation quintile, and comorbidities. Permission request in progress.
Transmission of SARS-CoV-2
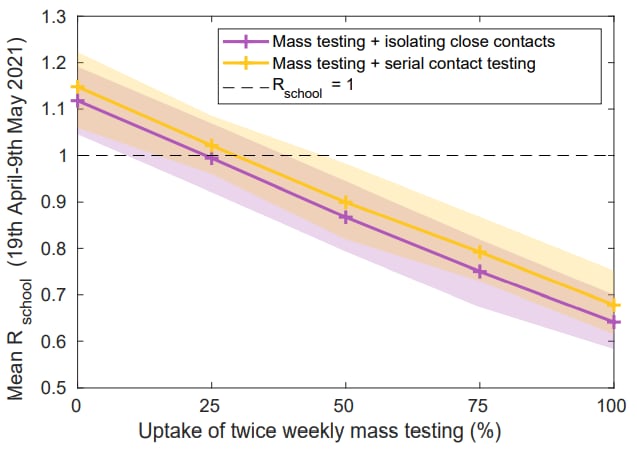
- Leng et al. Quantifying within-school SARS-CoV-2 transmission and the impact of lateral flow testing in secondary schools in England.external icon medRxiv (Preprint; July 16, 2021). Published in Nature Communications as Quantifying pupil-to-pupil SARS-CoV-2 transmission and the impact of lateral flow testing in English secondary schoolsexternal icon (March 1, 2022). Among secondary-school children in England (aged 11–16 years) who were tested for SARS-CoV-2 twice weekly with a lateral flow test between Aug 2020 and May 2021, the within-school R0 remained <1. A strategy of serial contact testing alongside mass testing is predicted by a stochastic model to substantially reduce absences compared to isolating close contacts, with only a marginal increase in within-school transmission.
Note: Adapted from Leng et al. The impact of regular mass testing uptake on within-school transmission. Shown are the predicted results of a strategy of twice weekly mass testing and isolation of close contacts (purple) vs twice weekly mass testing and serial contact testing (yellow). Shaded intervals represent 95% prediction intervals. Permission request in progress.
Natural History of SARS-CoV-2 Infection
- Jensen et al. Emergence of the E484K mutation in SARS-COV-2-infected immunocompromised patients treated with bamlanivimab in Germanyexternal icon. The Lancet Regional Health – Europe (July 14, 2021). After bamlanivimab (LY-CoV555) treatment, 5 of 6 immunocompromised COVID-19 patients tested positive for SARS-CoV-2 with the E484K mutation, which was not present before treatment. Of these 5 individuals, 1 patient further developed the E484Q mutation before reverting to E484K.
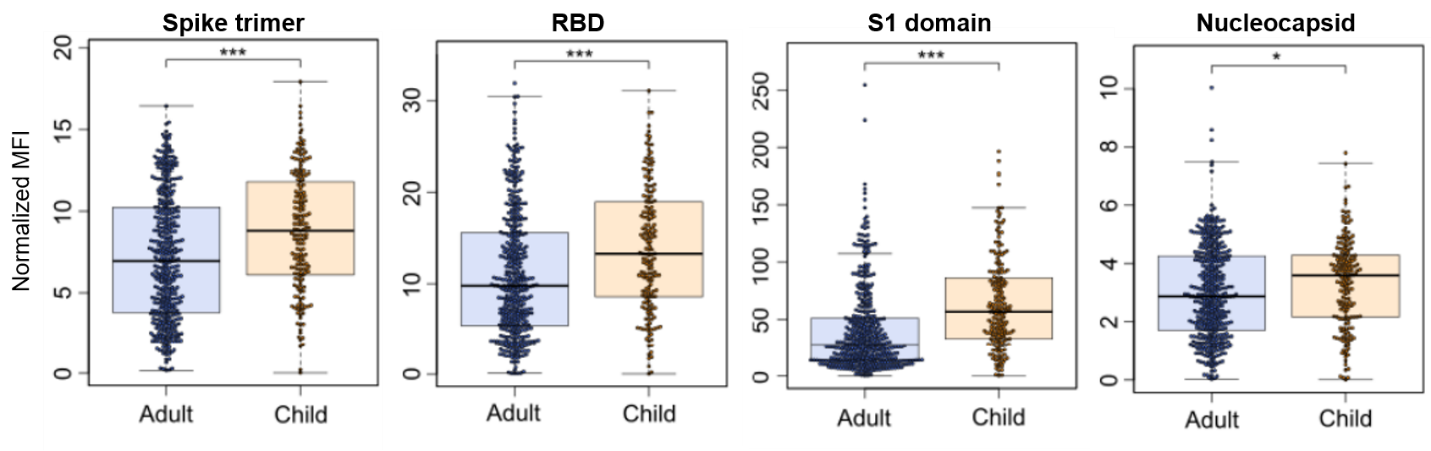
- Renk et al. Typically asymptomatic but with robust antibody formation: Children’s unique humoral immune response to SARS-CoV-2.external icon medRxiv (Preprint; July 22, 2021). While exposed children were less likely to seroconvert (33.76%, n = 548) than adults (57.88%, n = 717), they had stronger antibody responses and were 5 times more likely to be asymptomatic. Children’s antibodies persisted longer than adults’: 96.22% were seropositive 11–12 months post-infection compared to 82.89%.
Note: Adapted from Renk et al. Box and whisker plots showing antibody levels in adults and children against SARS-CoV-2 spike trimer, RBD, S1 domain, and nucleocapsid at 3–4 months post-initial infection within a household. * p<0.01, *** p<0.001 by Mann-Whitney-U (2-sided) test. Used by permission of author.
Prevention, Mitigation, and Intervention Strategies
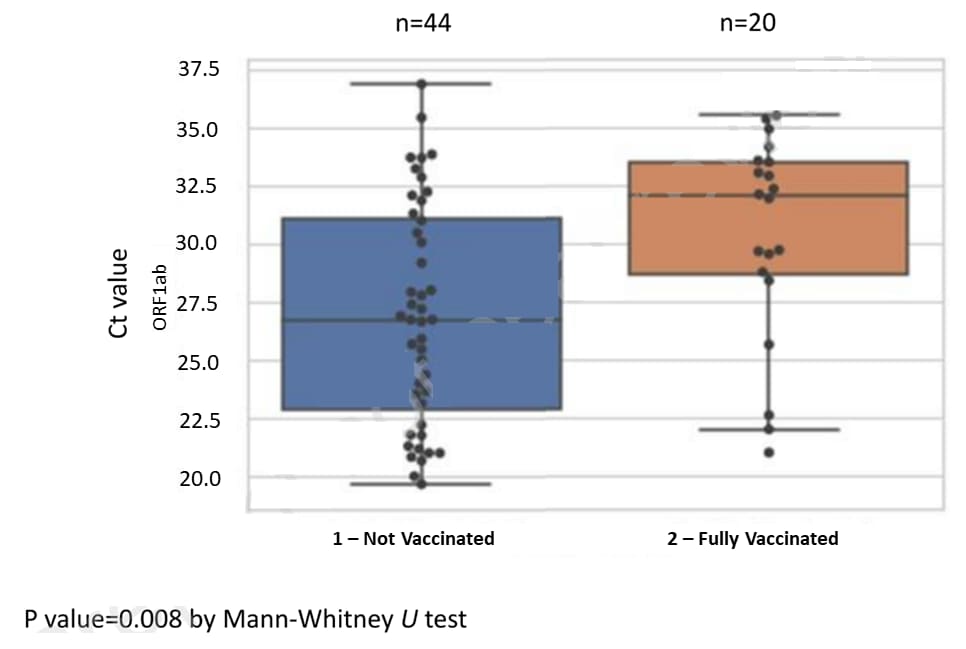
- Muhsen et al. Effectiveness of BNT162b2 mRNA COVID-19 vaccine against acquisitions of SARS-CoV-2 among health care workers in long-term care facilities: a prospective cohort study.external icon SSRN (Preprint; July 19, 2021). Published in Clinical Infectious Diseasesexternal icon (October 26, 2021). Among 9,162 health care workers (HCW, 6,960 vaccinated and 2,202 unvaccinated) at long-term care facilities in Israel, vaccine effectiveness following 2 doses of BNT162b2 was 89% (95% CI 83-93%). Median PCR Ct values among SARS-CoV-2 positive HCWs were lower in unvaccinated (26.7) compared to vaccinated (32.0) persons.
Note: Adapted from Muhsen et al. Box plots of Cycle threshold values from PCR test targeting SARS-CoV-2 ORF1ab gene among unvaccinated and BNT162b2 fully vaccinated individuals. Line in box is median, lower bound of box 25th percentile, upper bound of box 75th percentile, lowest point is minimum, and highest point is maximum. Each circle represents Ct value of 1 participant. Permission request in progress.
- Woldemeskel et al. The BNT162b2 mRNA vaccine elicits robust humoral and cellular immune responses in people living with HIVexternal icon. Clinical Infectious Diseases (July 22, 2021). Among people without evidence of prior infection, 7 to 17 days after 2nd dose of BNT162b2 T-cell immune response and neutralizing antibody response were similar in people living with HIV (n = 12) and in healthy donors (n = 17).
Social, Behavioral, and Communication Science
- Halbrook et al. Longitudinal assessment of COVID-19 vaccine acceptance and uptake among frontline medical workers in Los Angeles, California.external icon Clinical Infectious Diseases (July 22, 2021). Between September 2020 and February 2021, vaccine confidence increased among Los Angeles healthcare workers (n = 858) from 46.4% to 90.0%, and among first responders (n = 465) from 34.6% to 75.7%. Important factors cited by respondents were reports about Phase 3 efficacy and low side effects. Those remaining hesitant cited insufficient information on novel vaccines.
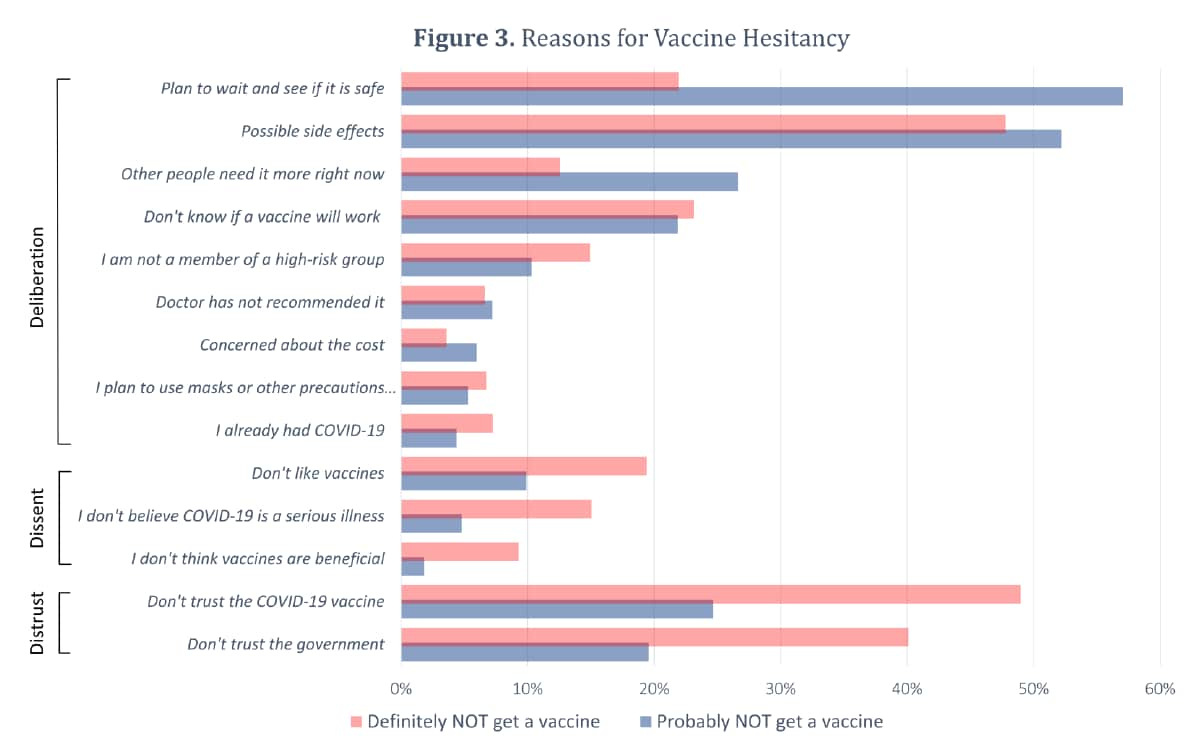
- Tram et al. Deliberation, dissent, and distrust: Understanding distinct drivers of COVID-19 vaccine hesitancy in the United States.external icon Clinical Infectious Diseases (July 16, 2021). Based on data from the US Census Bureau Pulse Survey (n = 459,235), vaccine uptake increased from 7.7 to 47% between January 6 and March 29, 2021. Vaccine hesitancy remained relatively steady at 10.2% (probably) and 8.2% (definitely) wouldn’t get vaccinated. Among those who were hesitant, reasons for vaccine rejection (definitely wouldn’t) were related to dissent and distrust while those reluctant (probably wouldn’t) reported reasons deliberative in nature.
Note: Adapted from Tram et al. Reasons for vaccine hesitancy by vaccine intention by “Definitely will not” and “Probably will not” receive the vaccine. Reasons are grouped by category (Distrust, Dissent, and Deliberation). Reproduced by permission of Oxford University Press on behalf of the Infectious Diseases Society of America. Please visit: https://academic.oup.com/cid/advance-article/doi/10.1093/cid/ciab633/6323151external icon.
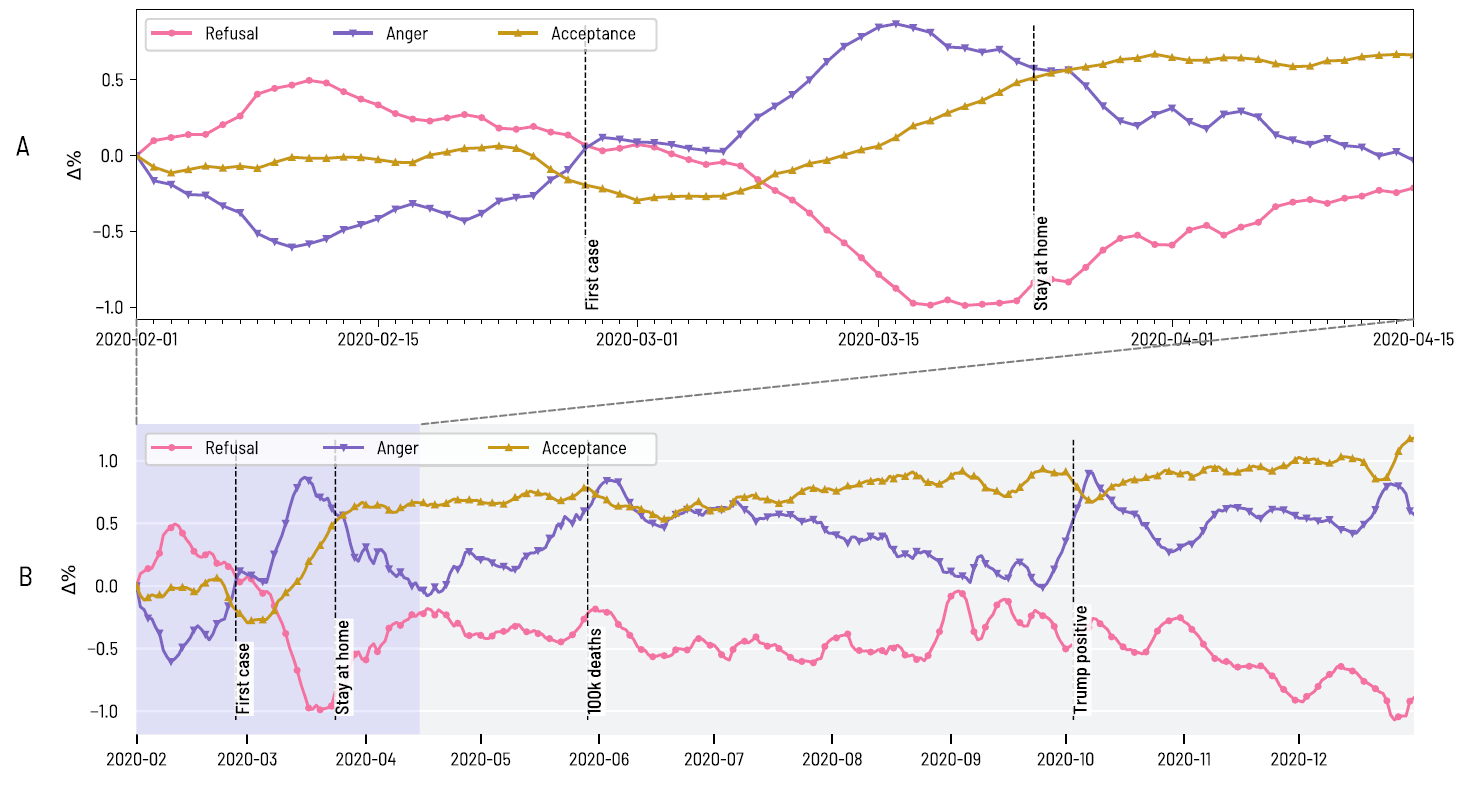
- Aiello et al. How epidemic psychology works on Twitter: evolution of responses to the COVID-19 pandemic in the U.S.external icon Humanities & Social Science Communications (July 23, 2021). Language about the COVID-19 pandemic in US tweets between February 1 and December 31, 2020, mirrored the stages of grief (refusal, anger, and acceptance). In the anger phase, outrage was expressed primarily against foreigners, political opponents, and people who adopted behavioral mitigations (e.g. masking). The acceptance phase was dominated by grief and mourning at the loss of so many thousands of people.
Note: Adapted from Aiello et al. Aggregated measures of the 3 phases of Epidemic Psychology over time. The evolution of language categories associated with Refusal, Anger, and Acceptance, from the first contagion wave in 2020 (A) and during all 3 waves in 2020 (B). Licensed under CC-BY.
- Simon et al. Trends in mortality from COVID-19 and other leading causes of death among Latino vs White individuals in Los Angeles County, 2011-2020.external icon JAMA (July 19, 2021). COVID-19 increased the age-adjusted mortality ratio of Latinos living in Los Angeles County from 516/100,000 during 2011–2019, which was lower than for Whites (630.3/100,000), to 741.7/100,000 in 2020, which was higher than for Whites (699.0/100,000).
Note: Adapted from Simon et al. Age-adjusted mortality rates among Latino and Non-Latino White residents of Los Angeles County, 2011–2020. Trend lines based on an analysis of 465,389 death certificate records. Age-adjusted to 2000 US standard population. Error bars indicate 95% CIs. Permission request in progress. Reproduced with permission from JAMA, 2021. Published online July 19, 2021. https://doi.org/ 10.1001/jama.2021.11945. Copyright© 2021 American Medical Association. All rights reserved.
Disclaimer: The purpose of the CDC COVID-19 Science Update is to share public health articles with public health agencies and departments for informational and educational purposes. Materials listed in this Science Update are selected to provide awareness of relevant public health literature. A material’s inclusion and the material itself provided here in full or in part, does not necessarily represent the views of the U.S. Department of Health and Human Services or the CDC, nor does it necessarily imply endorsement of methods or findings. While much of the COVID-19 literature is open access or otherwise freely available, it is the responsibility of the third-party user to determine whether any intellectual property rights govern the use of materials in this Science Update prior to use or distribution. Findings are based on research available at the time of this publication and may be subject to change.