COVID-19 Science Update released: June 30, 2020 Edition 26

The COVID-19 Science Update summarizes new and emerging scientific data for public health professionals to meet the challenges of this fast-moving pandemic. Weekly, staff from the CDC COVID-19 Response and the CDC Library systematically review literature in the WHO COVID-19 databaseexternal icon, and select publications and preprints for public health priority topics in the CDC Science Agenda for COVID-19 and CDC COVID-19 Response Health Equity Strategy.
Here you can find all previous COVID-19 Science Updates.
PEER-REVIEWED
SARS-CoV-2 positivity rate for Latinos in the Baltimore-Washington, DC regionexternal icon. Martinez et al. JAMA Network (June 18, 2020).
Key findings:
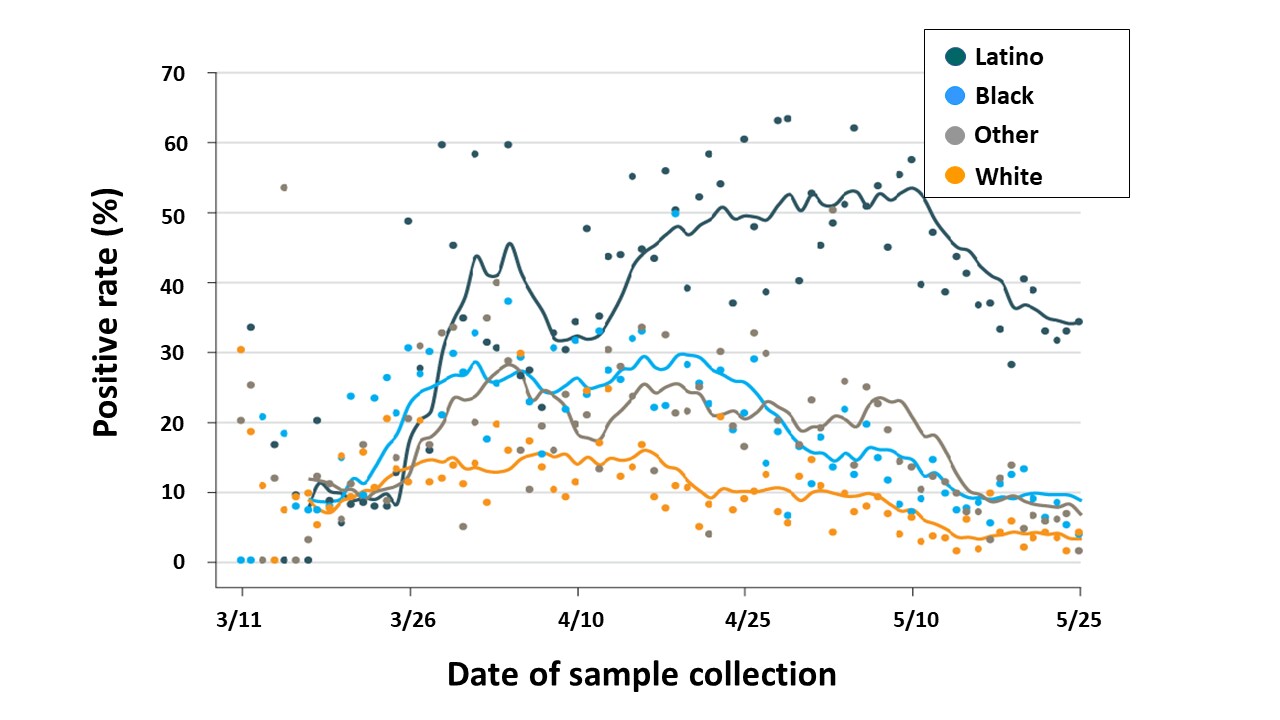
- Rates of SARS-CoV-2-RNA-positive NP swab were 43% among Latino patients, 18% among African American/black patients, 9% among White patients, and 17% among patients of other races/ethnicities.
- 61.5% of Latino patients aged 18–44 years who tested had a positive NP swab.
- Testing volume increased over time and rate of positive tests declined (Figure).
- Latino patients with infection were less likely to be hospitalized (29%) compared with White (40%) and African-American/black (42%) patients, possibly due to younger age and fewer comorbidities.
Methods: 37,727 patients were tested for SARS-CoV-2 at 5 hospitals and 30 outpatient clinics, Baltimore, MD, March 11–May 25, 2020. Limitations: Unclear if high positive rate among Latinos was due to low relative testing rates, high viral prevalence, or both.
Implications: High RT-PCR positivity was observed among Latinos in Baltimore. Addressing needs of Latino communities may prevent infection and address disparities.
Figure:
Note: Adapted from Martinez et al. Rates of positive PCR tests for SARS-CoV-2 over time by self-reported race/ethnicity. Reproduced with permission from JAMA. doi:10.1001/jama.2020.11374. Copyright©2020 American Medical Association. All rights reserved.
Outbreak investigation of COVID-19 among residents and staff of an independent and assisted living community for older adults in Seattle, Washingtonexternal icon. Roxby et al. JAMA Internal Medicine (May 21, 2020).
Key findings:
- After 2 residents were hospitalized for COVID-19, an older adult care facility initiated universal testing.
- 41% of residents and 28% of staff reported COVID-19-like symptoms at time of testing.
- 3 infected residents (all asymptomatic) and 2 staff members (both mildly symptomatic) were identified.
- At retesting a week later, 1 additional asymptomatic, infected resident identified.
- Other inventions included physical distancing, visitor bans, and enhanced disinfection.
- These efforts subsequently controlled transmission; no new infections were diagnosed.
Methods: All 80 residents and all 62 staff in an independent/assisted living community screened for symptoms and tested for SARS-CoV-2 by RT-PCR, Seattle, March 2020. Residents re-tested 7 days later. Limitations: Half of residents lived independently, may not be generalizable to all older adult care facilities.
Implications: Universal SARS-CoV-2 testing (rather than symptom screening) and enhanced infection control measures can halt transmission in older adult residential facilities.
Clinical and immunological assessment of asymptomatic SARS-CoV-2 infectionsexternal icon. Long et al. Nature Medicine (June 18, 2020).
Key findings:
- SARS-CoV-2 RNA levels at the first positive RT-PCR test among asymptomatic individuals were similar to levels observed in mildly symptomatic individuals (sex-, age-, comorbidity-matched), (see Limitations).
- Viral RNA was detectable ~5 days longer among asymptomatic group (median=19 days) than among matched symptomatic group (median=14 days), (see Limitations).
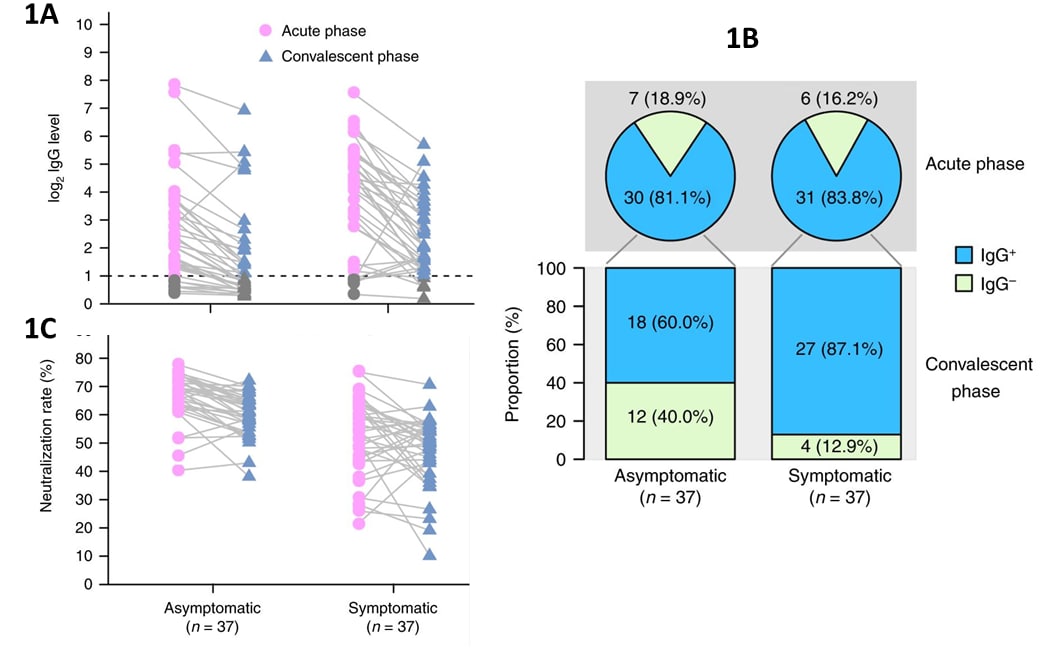
- IgG levels were lower in the asymptomatic group than the symptomatic group during infection and after viral clearance (Figure 1A).
- IgG levels had declined in both the asymptomatic and symptomatic groups when their IgG level were re-assessed about 8 weeks later (Figure 1A)
- Neutralizing antibody levels decreased in 81% (30/37) of asymptomatic and 62% (23/37) of symptomatic individuals (Figure 1C).
- Over 15% of both symptomatic and asymptomatic persons were seronegative at the initial assessment, which about 8 weeks later had declined slightly in symptomatic persons but doubled in asymptomatic persons (Figure 1B).
Methods: 37 individuals with asymptomatic SARS-CoV-2 infection were compared with 37 sex-, age-, and comorbidity-matched symptomatic individuals. Limitations: Case-finding (and possible stages of illness) differed between groups; groups were not matched on time since exposure; individuals were classified as asymptomatic if “relevant” symptoms (not otherwise defined) were absent; viral RNA detection may not equate with infectiousness.
Implications: IgG levels declined in the 8 weeks following infection, raising concern about a potential lack of durable post-infection immunity. Cautious interpretation of data is encouraged: relationships between detectable IgG, IgG levels, and immunity are unclear. Robust long-term longitudinal studies are needed to better understand post-infection immunity.
Figure:
Note: Adapted from Long et al. (A) Changes in acute and early convalescent virus-specific IgG (reported as log2 values). (B) The proportion of IgG seropositive participants in the acute phase (viral RNA is detectable) and convalescent phase (8 weeks after viral RNA is no longer detectable). (C) Neutralizing acute and early convalescent antibody activity (% neutralization rate) for asymptomatic and symptomatic individuals from the acute phase (viral RNA is detectable) and early convalescent phase. Permission request in process. Reprinted by permission from Springer Nature Customer Service Centre GmbH: Springer Nature, Long, Q., Tang, X., Shi, Q. et al. Clinical and immunological assessment of asymptomatic SARS-CoV-2 infections. Nat Med 26, 1200–1204 (2020). https://doi.org/10.1038/s41591-020-0965-6external icon. COPYRIGHT 2020.
New onset neurologic events in people with COVID-19 infection in three regions in China. external iconXiong et al. Neurology (June 24, 2020).
Key findings:
- Among 917 hospitalized COVID-19 patients, 32 (3.5%) developed serious neurologic conditions (impaired consciousness=22; stroke=7; both=3).
- 30 (9.4%) of 319 patients with severe-critical COVID-19 developed these neurologic conditions.
- SARS-CoV-2 not found in CSF of 3 who underwent lumbar puncture.
- Patients age > 60 years were more likely than younger patients to develop new neurologic conditions.
Methods: Assessment of serious neurologic events among COVID-19 patients from 56 hospitals, Sichuan, Wuhan, and Chongqing, China, between January 18 to March 20, 2020. Limitations: Few children included; some patients still hospitalized at end of study, possibly under-estimating neurologic event incidence.
Implications: Patients with severe COVID-19, especially those age >60 years, may be at high risk of stroke and/or impaired consciousness.
In late March 2020, jurisdictions across the US began issuing stay-at-home orders to control COVID-19 spread and limit strain on the healthcare system. Many healthcare providers and hospitals limited the range of services provided. In the following months, visits for routine immunizations and to emergency departments plummeted. The following papers describe changes in emergency medical services during this time period.
PEER-REVIEWED
A. Effect of the coronavirus disease 2019 (COVID‐19) pandemic on the United States emergency medical services system: a preliminary reportexternal icon. Lerner et al. Academic Emergency Medicine (June 17, 2020).
Key findings:
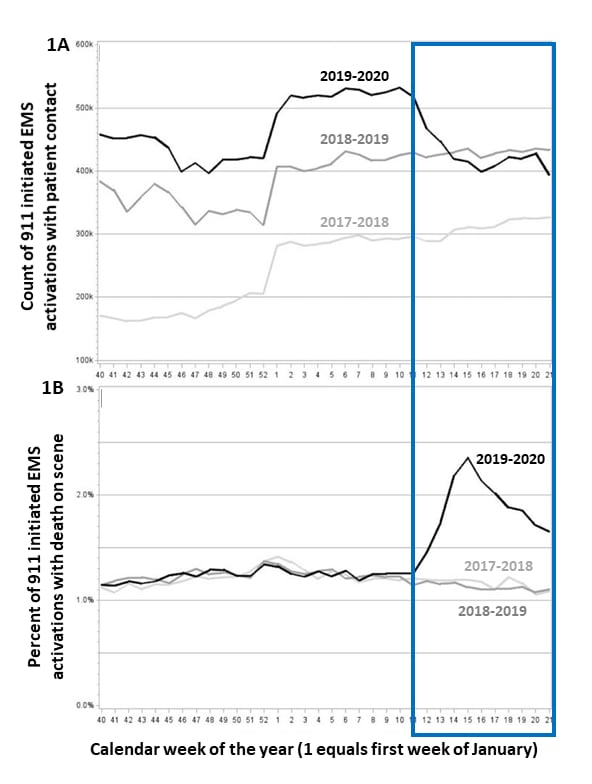
- Emergency medical services (EMS) responses decreased 25% during March–May 2020 compared with the same period in previous years (Figure 1A).
- During the same period, EMS-attended deaths in the field doubled (Figures 1B).
Methods: Analysis of National EMS Information System (NEMSIS) data for EMS care, October–May, 2018–20. Limitations: States in NEMSIS increased 33% during study period; unknown causes of death.
Figure:
Note: Adapted from Lerner et al. Number of EMS responses decreased (Figure 1A) while EMS-attended deaths (Figure 1B) increased during March (week 11) through May (week 21), blue box, compared to the same time period in 2018 and 2019. Available via Wiley Public Health Emergency Collection through PubMed Central.
B. Characteristics associated with out-of-hospital cardiac arrests and resuscitations during the novel coronavirus disease 2019 pandemic in New York Cityexternal icon. Lai et al. JAMA Cardiology (June 19, 2020).
Key findings:
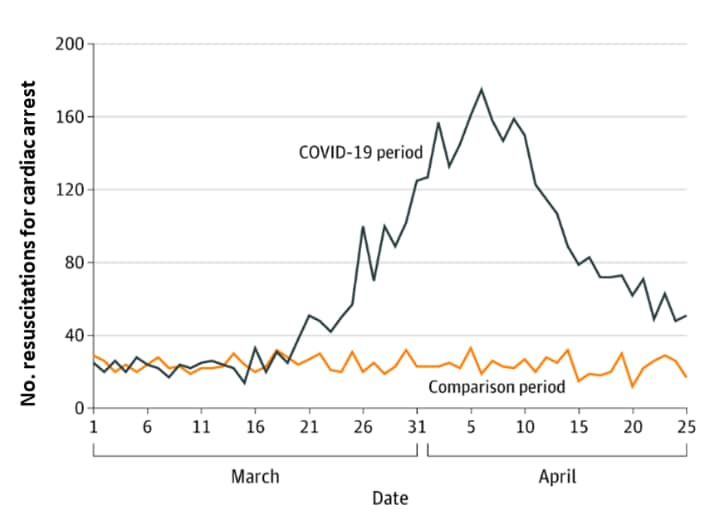
- Number of out-of-hospital resuscitations for cardiac arrests tripled during the pandemic compared with the same period in 2019 (Figure).
- Patients who underwent resuscitation for cardiac arrest in 2020 were more likely to be older, non-white, have comorbidities, and less likely to be successfully resuscitated than in 2019.
Methods: Analysis of New York City 911 EMS data, March 1–April 25, 2019 and 2020. Limitations: Unknown if deaths caused by COVID-19.
Figure:
Note: Adapted from Lai et al. EMS-attended cardiac arrests in NYC during the COVID-19 pandemic compared with 2019. Licensed under CC-BY.
Implications of Lerner et al. and Lai et al.: These data strongly suggest that persons with life-threatening emergencies delayed seeking timely care, perhaps as a result of stay-at-home orders or fear of exposure to SARS-CoV-2 in the healthcare system, resulting in increased out-of-hospital and EMS-attended deaths.
PEER-REVIEWED
Nosocomial transmission of COVID-19: a retrospective study of 66 hospital-acquired cases in a London teaching hospitalexternal icon. Rickman et al. Clinical Infectious Diseases (June 20, 2020).
Key findings:
- Of 435 SARS-CoV-2-PCR-positive inpatients, 66 (15%) were infected in the hospital (47 definite and 19 probable hospital-acquired cases).
- Of patients with hospital-acquired infections, 55% had shared a room with an infected patient, 14% had been on the same floor as an infected patient and 31% had had no clear contact with infected patients.
- The percentage of hospital-acquired infections decreased from 21% to 7% after visitor restrictions, staff testing, and cohorting policies were implemented.
Methods: Patients admitted to one of four London hospitals, between March 2 and April 12, 2020. A case was defined as hospital-acquired if onset of COVID-19 symptoms occurred >14 days (definite) or >7 days (probable) after admission. Limitations: Only symptomatic inpatients were tested for SARS-CoV-2, which may underrepresent asymptomatic transmissions and infections; unclear whether hospital staff were tested.
Implications: Hospital-acquired transmission can occur without proper infection prevention and control measures.
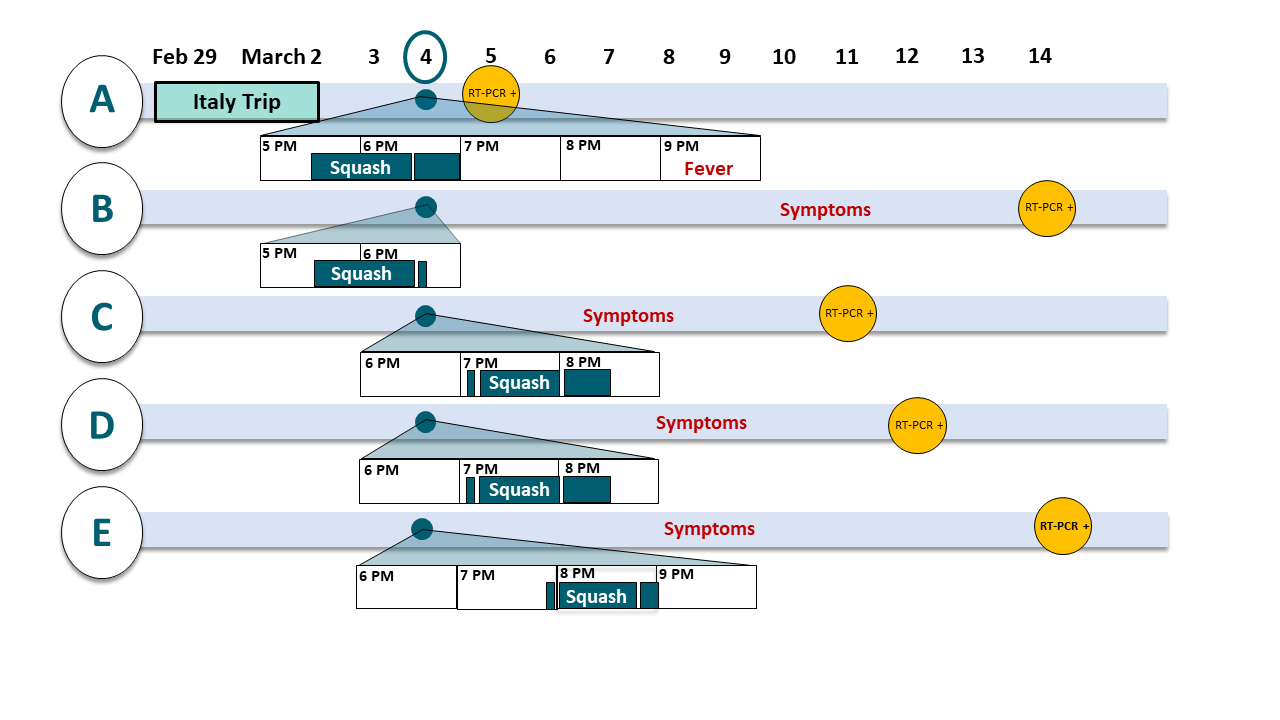
Possible indirect transmission of COVID-19 at a squash court, Slovenia, March 2020: case reportpdf iconexternal icon. Brlek et al. Epidemiology & Infection (June 25, 2020).
Key findings:
- Investigation of recent traveler with SARS-CoV-2 infection (case A, diagnosed March 5) identified 4 more persons with SARS-CoV-2, all of whom played squash at the same court on March 4, 2020.
- A was pre-symptomatic, played against B during 5:30-6:30 pm; A & B left by 7:00 (Figure).
- C & D played from 7:15–8:00 pm, then spoke with case E, showered, and left by 8:30. Case E played against a non-case from 8:00–8:45 pm (Figure).
- All five persons played on the same squash court but did not share equipment.
- Person A developed symptoms hours after the game on March 4, 2020.
- Persons B, C, D, and E developed symptoms and tested positive in subsequent days (Figure).
- No other contact between cases were reported.
Methods: Investigation of SARS-CoV-2 cases, Slovenia, March, 2020. Limitations: Community transmission was possible but thought to be unlikely at the time; viral genotyping was unavailable to help support the epidemiologic evidence that these infections were related.
Implications: SARS-CoV-2 was likely transmitted through fomites or aerosols under unique circumstances that amplified transmission risk: intense and prolonged respiratory droplet generation in a small poorly ventilated space from a person on the cusp of illness onset.
Figure:
Note: Adapted from Brlek et al. Figure displays the timeline of contact, symptom onset, and RT-PCR results of 5 persons (A-E) infected with SARS-CoV-2. Light blue horizontal bars show dates of A’s travel, and A-E’s symptom onset and positive RT-PCR. White horizontal bars show March 4 attendance at the squash court venue (dark teal bars) and the times spent playing squash (dark teal bars reading “Squash”). Licensed under CC-BY 4.0.
PREPRINTS (NOT PEER-REVIEWED)
Effect of dexamethasone in hospitalized patients with COVID-19 – Preliminary report.external icon Horby et al. medRxiv (June 22, 2020). Published external iconin NEJM (July 17, 2020).
Key findings:
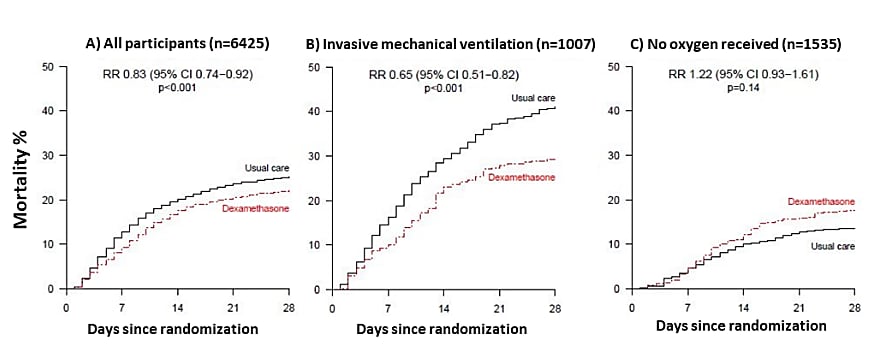
- 21.6% of COVID-19 patients allocated to dexamethasone died within 28 days vs. 24.6% of control patients (risk ratio [RR] 0.83 [95% CI 0.74–0.92]) (Figure 1A).
- Deaths were reduced 35% among intubated patients (RR 0.65 [95% CI 0.51–0.82]) (Figure 1B).
- No benefit was observed for patients not requiring supplemental oxygen (Figure 1C).
- Patients treated with dexamethasone were slightly more likely to be discharged by 28 days (64.6%) than control patients (61.1%, p=0.002).
Methods: Randomized open-label trial (RECOVERY) that compared dexamethasone plus standard care (2,104) vs. standard care (4,321) in hospitalized COVID-19 patients, 176 hospitals, UK, between March 9 and June 8, 2020. Limitations: No safety data; non-blinded.
Implications: Dexamethasone reduced mortality among hospitalized COVID-19 patients who required supplemental respiratory support and oxygen.
Figure:
Note: Adapted from Horby et al. 28−day mortality in all patients (A), patients receiving mechanical ventilation (B) and not receiving oxygen support (C) at randomization. RR-age adjusted rate ratio. Licensed under CC-BY 4.0.
Hyperinflammation and cytokine storm — destructive human immune responses to SARS-CoV-2 infection— may cause rapid deterioration of patients. Treatments, such as monoclonal antibodies, that dampen inflammatory responses and are used to treat autoimmune diseases, may prove effective for severe COVID-19. Mavrilimumab, which inhibits granulocyte-macrophage colony-stimulating factor (GM-CSF) activity, and tocilizumab, which inhibits IL-6, were examined as potential treatment for patients with severe COVID-19. Another group of monoclonal antibodies, immune checkpoint inhibitors (ICIs) used in treatment of several types of cancer can boost the immune response.
PEER-REVIEWED
A. GM-CSF blockade with mavrilimumab in severe COVID-19 pneumonia and systemic hyperinflammation: a single-centre, prospective cohort studyexternal icon. De Luca et al. Lancet Rheumatology (June 16, 2020).
Key findings:
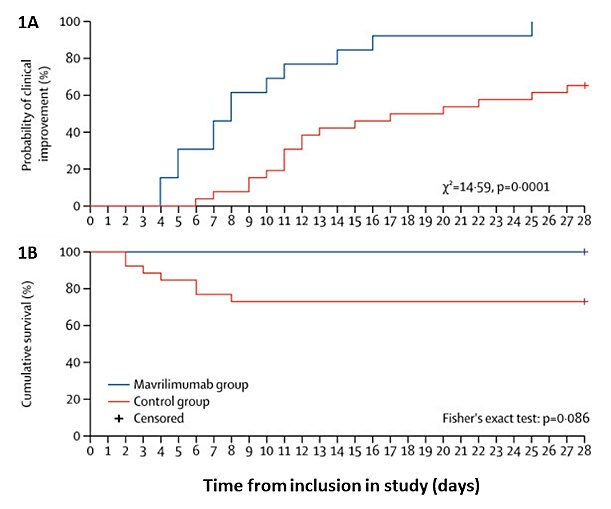
- Non-randomized patients with severe COVID-19 and hyperinflammation treated with mavrilimumab were likely to experience clinical improvement (100% vs. 65%) and that was faster (8 vs. 19 days) and to experience a shorter time to hospital discharge (10 vs. 20 days) than control patients (Figure 1A).
- No mavrilimumab-treated patients died; 7 (27%) control patients died (p=0.09) (Figure 1B).
Methods: 39 non-intubated patients with severe COVID-19 pneumonia and hyperinflammation (13 received IV mavrilimumab), March 17–April 15, 2020, Milan, Italy. Control patients (26) were eligible for, but not treated with mavrilimumab because of lack of available drug (24) or consent (2). Standard therapy included hydroxychloroquine, O2, ± azithromycin. Limitations: Non-randomized.
Figure:
Note: Adapted from De Luca et al. Time to clinical improvement (A) and cumulative survival rate (B) by day 28 in the mavrilimumab and control groups. This article was published in Lancet Rheumatology, Vol 2, De Luca et al., GM-CSF blockade with mavrilimumab in severe COVID-19 pneumonia and systemic hyperinflammation: a single-centre, prospective cohort study, Page e465-e473, Copyright Elsevier 2020. This article is currently available at the Elsevier COVID-19 resource center: https://www.elsevier.com/connect/coronavirus-information-centerexternal icon.
B. Tocilizumab in patients with severe COVID-19: a retrospective cohort studyexternal icon. Guaraldi et al. Lancet Rheumatology (June 24, 2020).
Key findings:
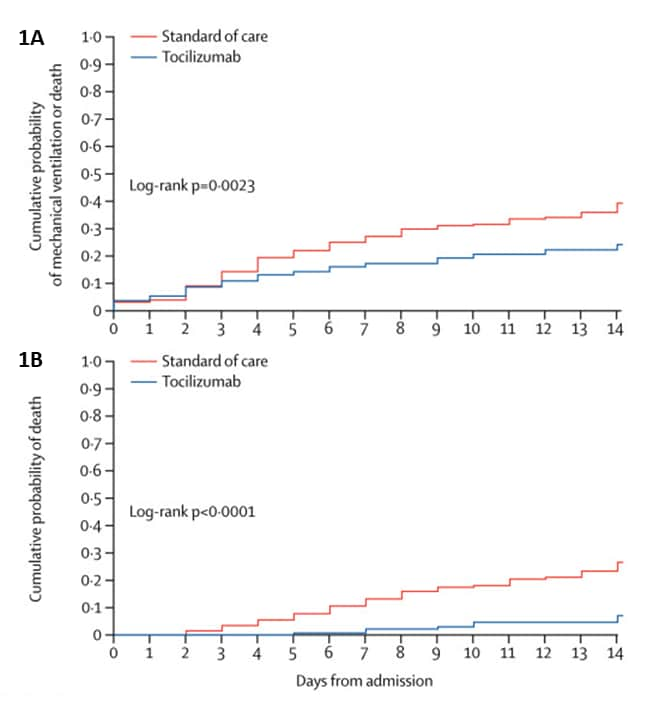
- In a non-randomized study, tocilizumab was associated with reduced risk of mechanical ventilation or death (adjusted hazard ratio [aHR] 0.61, 95% CI 0.40–0.92) (Figure 1A).
- Risk of death was lower in the tocilizumab-treated group (aHR 0.38, 0.17–0.83) (Figure 1B).
- 24 (13%) tocilizumab-treated patients were diagnosed with new bacterial or fungal infections or viral (HBV, HSV-2) reactivation vs. 14 (4%) of patients treated with standard therapy (p<0.001).
- Results did not differ by route of tocilizumab administration.
Methods: Observational study that compared 179 adults with severe COVID-19 pneumonia treated with tocilizumab (intravenously [n=88] or subcutaneously [n=91]) plus standard therapy to 365 hospitalized patients treated with standard therapy. Risk of mechanical ventilation or death was adjusted for demographics, symptom duration, and illness severity (SOFA score). Limitations: Non-randomized, baseline differences in groups (less severe disease in comparison group); 2 different tocilizumab regimens; glucocorticoids started in 30% of tocilizumab group and 17% of comparison group.
Figure:
Note: from Guaraldi et al. Cumulative probability of mechanical ventilation or death (Figure 1A) and death (Figure 1B) for tocilizumab-treated patients and patients who only received standard therapy. This article was published in Lancet Rheumatology, Vol 2, Guaraldi et al., Tocilizumab in patients with severe COVID-19: a retrospective cohort study, Page e474-e484, Copyright Elsevier 2020. This article is currently available at the Elsevier COVID-19 resource center: https://www.elsevier.com/connect/coronavirus-information-centerexternal icon.
C. Determinants of COVID-19 disease severity in patients with cancerexternal icon. Robilotti et al. Nature Medicine (June 18, 2020).
Key findings:
- Among 423 patients undergoing cancer treatment and with SARS-CoV-2 infection, 168 (40%) were hospitalized; 87 developed severe respiratory illness, 51 died within 30 days.
- After multivariable adjustment, cancer treatment with immune checkpoint inhibitors (ICIs) was independently associated with hospitalization (aOR 2.84, 95% CI 1.24–7.72) and severe respiratory illness (aOR 2.74, 95% CI 1.37–5.46).
Methods: Retrospective analysis of 423 patients with cancer and SARS-CoV-2 infection, Memorial Sloan Kettering Cancer Center, NYC, March 10–May 7, 2020. Severe respiratory illness classified if required high-flow oxygen or mechanical ventilation. Limitations: Small study; single center.
Implications of 3 studies (De Luca et al., Guaraldi et al., & Robilotti et al.): Monoclonal antibodies directed at alteration of the immune response may affect the course of COVID-19. Tocilizumab treatment may reduce the risk of mechanical ventilation or death in patients with severe COVID-19 pneumonia. Mavrilimumab may improve outcomes in patients with severe COVID-19 and hyperinflammation. RCTs are needed to confirm these findings. Persons receiving ICIs for cancer treatment might be at elevated risk for severe COVID-19.
- Hammarstrom et al. Development of passive immunity against SARS-CoV-2 for management of immunodeficient patients—a perspectiveexternal icon. Describes passive immunotherapy as a potential preventative measure for immunocompromised persons during the COVID-19 pandemic.
- Helgason et al. Beating the odds with systematic individualized care nationwide prospective follow-up of all patients with COVID-19 in Icelandexternal icon. Journal of Internal Medicine. Describes steps taken in Iceland that may have contributed to fewer hospital admissions and lower mortality than projected.
- Servick. Can phone apps slow the spread of the coronavirus? external iconScience. Reviews use of cellphone apps and their tracking capability for case identification and contact tracing.
- Leung et al. No detectable surge in SARS-CoV-2 transmission attributable to the April 7, 2020 Wisconsin electionexternal icon. American Journal of Public Health. Analyzes data from voting in Wisconsin on April 7 and concludes that it was a low risk activity.
- Wozniak et al. Involvement of cisgender and transgender individuals in studies on the impact of hormonal therapy on COVID-19external icon. AIDS Patient Care and STDs. A call to include transgender persons in COVID-19 research initiatives and data collection.
Disclaimer: The purpose of the CDC COVID-19 Science Update is to share public health articles with public health agencies and departments for informational and educational purposes. Materials listed in this Science Update are selected to provide awareness of relevant public health literature. A material’s inclusion and the material itself provided here in full or in part, does not necessarily represent the views of the U.S. Department of Health and Human Services or the CDC, nor does it necessarily imply endorsement of methods or findings. While much of the COVID-19 literature is open access or otherwise freely available, it is the responsibility of the third-party user to determine whether any intellectual property rights govern the use of materials in this Science Update prior to use or distribution. Findings are based on research available at the time of this publication and may be subject to change.