COVID-19 Science Update released: June 16, 2020 Edition 22

The COVID-19 Science Update summarizes new and emerging scientific data for public health professionals to meet the challenges of this fast-moving pandemic. Weekly, staff from the CDC COVID-19 Response and the CDC Library systematically review literature in the WHO COVID-19 databaseexternal icon, and select publications and preprints for public health priority topics in the CDC Science Agenda for COVID-19 and CDC COVID-19 Response Health Equity Strategy.
Here you can find all previous COVID-19 Science Updates.
Severe COVID-19 in children is rare. Case reports describe an emerging inflammatory syndrome with features of Kawasaki Disease and Toxic Shock Syndrome that may be related to COVID-19. This multisystem inflammatory syndrome in children (MIS-C) has also been termed pediatric multisystem inflammatory syndrome (PMIS) and pediatric inflammatory multisystem syndrome (PIMS). We summarize 5 articles describing MIS-C in hospitalized children in the US, UK, and France.
PEER-REVIEWED
A. SARS-CoV-2-related paediatric inflammatory multisystem syndrome, an epidemiological study, France, 1 March to 17 May 2020.external icon Belot et al. Eurosurveillance (June 4, 2020).
Key findings:
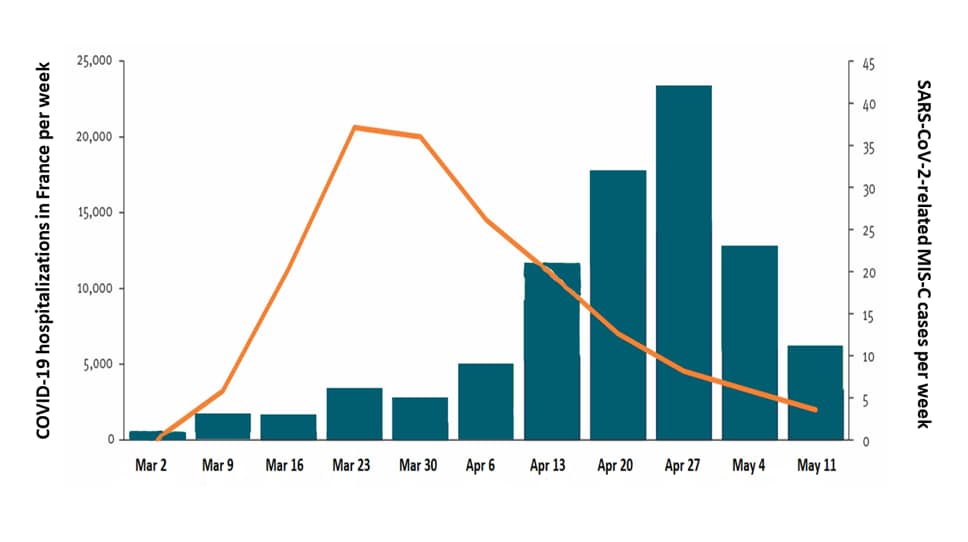
- SARS-CoV-2-related MIS-C cases increased 4–5 weeks after peak COVID-19 hospitalizations (Figure).
- Compared with 48 children with MIS-C without SARS-CoV-2, 108 hospitalized children with SARS-CoV-2-related MIS-C were:
- older (median 8 years vs. 3 years)
- more likely to have heart inflammation (myocarditis) (70% vs. 10%)
- more often critically ill (67% vs. 8%).
Methods: French nationwide surveillance for MIS-C cases, between March 1 to May 17, 2020. MIS-C defined as having at 1 or more symptoms of: inflammation of lining around the heart, lungs, or abdomen (serositis) or of heart muscle (myocarditis), symptoms of autoimmune macrophage activation syndrome, or Kawasaki-like disease. Evidence of SARS-CoV-2 defined by positive RT-PCR or serology test, direct link with a confirmed COVID-19 case, or suggestive radiography. Limitations: Use of non-standard case definition; definition of “SARS-CoV-2-related” did not require RT-PCR or IgG; unclear classification for 13 children.
Figure:
Note: Adapted from Belot et al. Weekly number of COVID-19 hospitalizations (right axis) and number of SARS-CoV-2-related MIS-C cases (left axis) in France, between March 1 to May 17, 2020. Licensed under CC-BY 4.0.
B. Kawasaki-like multisystem inflammatory syndrome in children during the COVID-19 pandemic in Paris, France: prospective observational study.external icon Toubiana et al. BMJ (June 3, 2020).
Key findings:
- Of 21 children and adolescents with MIS-C, median age 8 years, and 12/21 (57%) of African or Caribbean ancestry.
- Median interval between SARS-CoV-2 contact and MIS-C was 36 days (range 18–45).
- All had GI symptoms, 16/21 (76%) had heart inflammation (myocarditis); half met criteria for Kawasaki disease; all had elevated IL-6.
- 17/21 (81%) were critically ill, including 8 with sustained hypotensive shock; none died.
- 8/21 (38%) had positive SARS-CoV-2 RT-PCR, 19/21 (90%) had SARS-CoV-2 IgG.
Methods: Clinical case-series of children and adolescents ≤18 years admitted to single hospital in France, between April 27 and May 11, 2020 with Kawasaki disease. Some might be included in Belot et al. Limitations: Single center; small sample; limited to those with Kawasaki-like disease.
C. Clinical characteristics of 58 children with a pediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2external icon. Whittaker et al. JAMA (June 8, 2020).
Key findings:
- Among 58 children hospitalized with SARS-CoV-2-related MIS-C in England, all presented with fever, 31 (53%) with abdominal pain, 30 (52%) with diarrhea, and 30 (52%) with rash; 8 developed coronary artery aneurysms.
- 29/58 (50%) were critically ill: 27 were in shock, 25 required ventilation, 1 died.
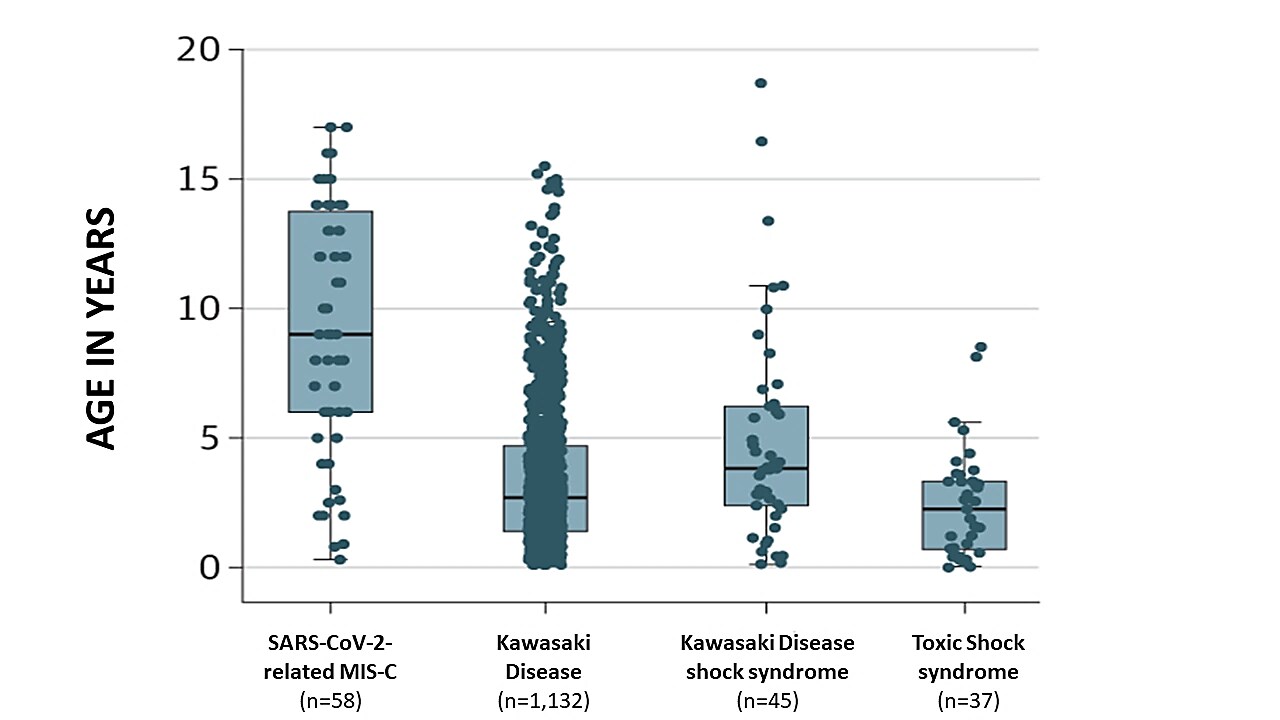
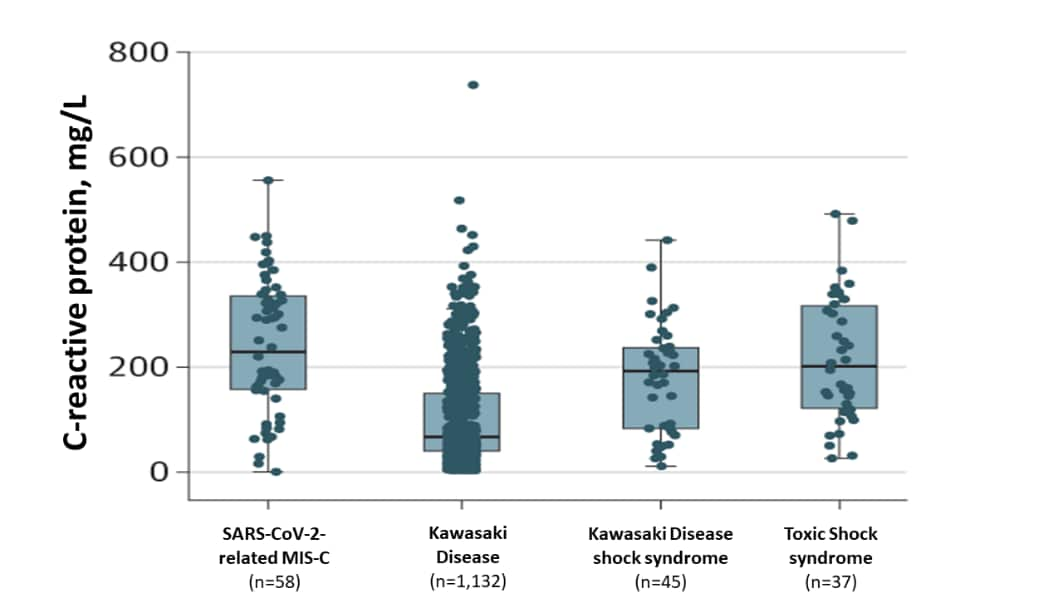
- Children with SARS-CoV-2-related MIS-C were older (Figure 1) and had a trend towards greater inflammation (measured by C-reactive protein) than children with other non-SARS-CoV-2-related inflammatory disorders (Figure 2).
- SARS-CoV-2 IgG detected in 40/46 (87%) tested.
Methods: Clinical case-series from 8 hospitals in England, between March 23 and May 16, 2020. Cases met criteria for WHO, UK, or US case MIS-C definitions, but SARS-CoV-2 exposure not required for inclusion. Limitations: Cases identified through voluntary surveys, may not include all MIS-C cases.
Figure 1
Note: Adapted from Whittaker et al. Ages of hospitalized children with different inflammatory disorders. Black horizontal lines within boxes represent median ages (years), top and bottom of boxes represent 25th and 75th percentiles, with 95% confidence intervals (thin black line “whiskers”). Reproduced with permission from JAMA. doi:10.1001/jama.2020.10369. Copyright©2020 American Medical Association. All rights reserved.
Figure 2
Note: Adapted from Whittaker et al. C-reactive protein (inflammation) levels of hospitalized children with different inflammatory disorders. Black horizontal lines within boxes represent median C-reactive protein levels, top and bottom of and boxes represent the 25th and 75th percentiles, with 95% confidence intervals (thin black line “whiskers”). Reproduced with permission from JAMA. doi:10.1001/jama.2020.10369. Copyright©2020 American Medical Association. All rights reserved.
D. Gastrointestinal symptoms as a major presentation component of a novel multisystem inflammatory syndrome in children (MIS-C) that is related to COVID-19: a single center experience of 44 cases.external icon Miller et al. Gastroenterology (June 4, 2020).
Key findings:
- Of 44 hospitalized children in a New York hospital with MIS-C, median age was 7 years.
- GI symptoms (e.g., pain, nausea/vomiting, diarrhea, bleeding) were reported by 37/44 (84%).
- 13 (30%) presented with fever and mild GI symptoms during the week prior to hospitalization.
- 11/44 (25%) required supplemental oxygen; 1 child was intubated; none died.
Methods: Clinical case-series of 44 hospitalized children and adolescents with MIS-C and exposure or evidence of current or recent SARS-CoV-2 infection, New York City, April 18–May 22, 2020. Limitations: MIS-C not defined; single center, may not be representative.
Multisystem inflammatory syndrome related to COVID-19 in previously healthy children and adolescents in New York Cityexternal icon. Cheung et al. JAMA (June 8, 2020).
Key findings:
- Of 17 hospitalized children and adolescents with SARS-CoV-2-related MIS-C, median age was 8 years (range 1.8–16); 12/17 (71%) were white; 3/17 (18%) had mild asthma.
- GI symptoms were reported by 14/17 (82%); 1 had acute bowel inflammation (ileocolitis).
- Moderate–severe cardiac dysfunction in 6/17 (35%); 1 had a coronary aneurysm.
- 15/17 (88%) were critically ill: 13 were in shock on presentation; none were intubated or died.
- IL-6 was elevated in 16/17 (94%).
Methods: Clinical case-series of 17 hospitalized children and adolescents with SARS-CoV-2-related MIS-C, New York City, April 18–May 5, 2020. Some patients might be included in Miller et al. Limitations: Single center, may not be representative.
Implications for 5 studies (Belot et al., Toubiana et al., Whittaker et al., Miller et al. & Cheung et al.): MIS-C can cause severe illness and seems to be a SARS-CoV-2 postinfectious complication. Patients with SARS-CoV-2-related MIS-C were older and required more intensive care than patients with Kawasaki disease. Pneumonia was noticeably absent; mechanical ventilation seemed to be used to support patients with cardiovascular collapse (shock) rather than respiratory failure. Early MIS-C with GI symptoms may be misdiagnosed as mild GI illness. Studies are needed to understand the spectrum of MIS-C severity, timing between SARS-CoV-2 infection and MIS-C, risk factors, possible long-term complications, and therapy.
PEER-REVIEWED
Psychological distress and loneliness reported by US adults in 2018 and April 2020external icon. McGinty et al. JAMA (June 3, 2020).
Key findings:
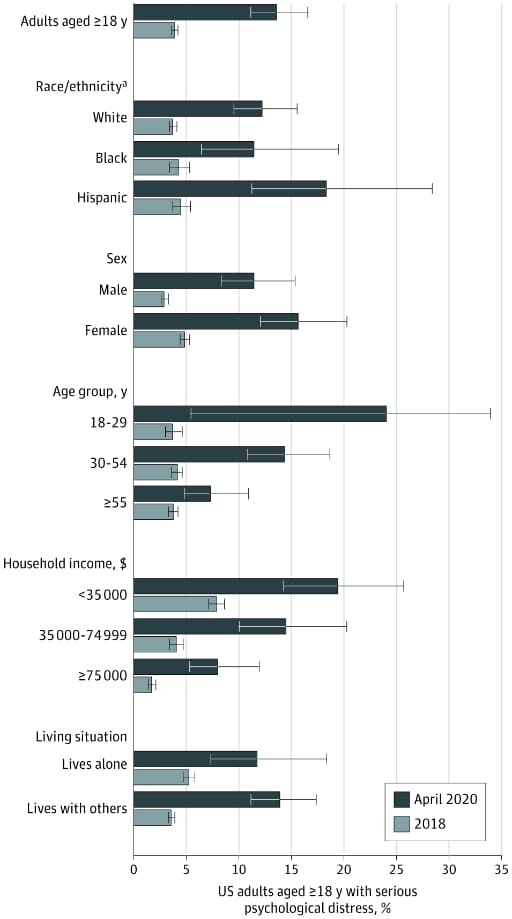
- Prevalence of serious psychological distress among US adults was higher in April 2020 (13.6%) than in 2018 (3.9%) (Figure).
- Distress was substantially higher than in 2018 among adults ages 18–29 years, Hispanic/Latinos, and those in low income households (Figure).
Methods: Prevalence of self-reported psychological distress among ~27,000 adults (1,468: April 2020; 25,417: 2018) from 2 surveys with same questions. Weighted for nationally representative estimates. Limitations: Two surveys might have sampled different populations; possible overestimates if those with distress were more likely to respond.
Implications: A large proportion of US adults reported serious psychological distress in April, 2020. Increased access to mental health and social support services, particularly for young adults, may be important.
Figure:
Note: From McGinty et al. Psychological distress among US adults aged ≥18 years overall and by group, April 2020 vs. 2018. April 2020 results (dark bars) are from the Johns Hopkins COVID-19 Civic Life and Public Health Survey. 2018 results (light bars) are from the National Health Interview Survey. Error bars show 95% CIs. Reproduced with permission from JAMA. doi:10.1001/jama.2020.9740. Copyright©2020 American Medical Association. All rights reserved.
Some patients with COVID-19 develop acute respiratory distress syndrome (ARDS), or extensive lung inflammation, in the second week of infection, characterized by low oxygen levels and severe shortness of breath. ARDS has 3 stages: exudative (fluid build-up, diffuse alveolar damage, and cellular debris lining the air sacs), proliferative (recovery and early scarring), and fibrotic (extensive scarring). Severe COVID-19 also causes abnormal clotting. These papers describe autopsy findings from adults who died from COVID-19.
PEER-REVIEWED
A. Pulmonary post-mortem findings in a series of COVID-19 cases from northern Italy: a two-centre descriptive studyexternal icon. Carsana et al. Lancet Infectious Diseases (June 8, 2020).
Key findings:
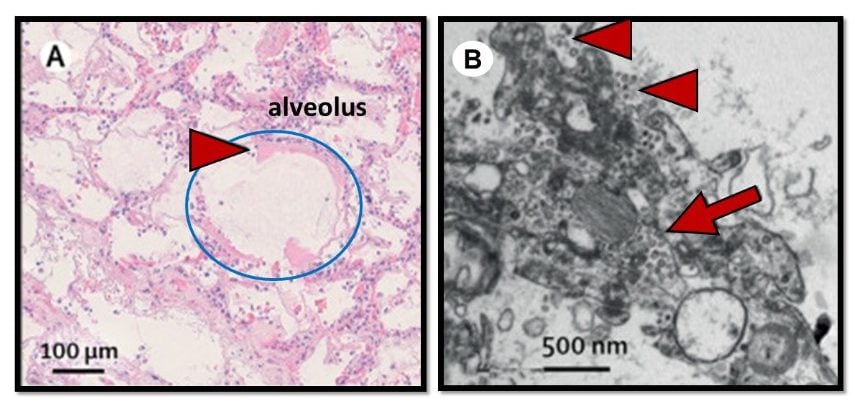
- Lungs from 38 Italian patients had extensive damage.
- All showed exudative and proliferative phases of ARDS; none had fibrotic scarring (Figure A).
- 33 (87%) also had diffuse small blood clots; none had bleeding.
- All lung samples had dead cells lining the air sacs (alveoli).
- 9/10 samples had viral-like particles in or around cells lining alveoli, suggesting viral infection of these cells (Figure B).
Methods: Postmortem examination of lung tissue from 38 patients who died from COVID-19 in 2 hospitals in northern Italy, between February 29 and March 24, 2020. Patients had pre-existing comorbidities including hypertension (in 18), cardiovascular disorders (11), diabetes (9), and mild chronic obstructive pulmonary disease (3). Limitations: Patient hearts were not examined; illness duration was not provided.
Figure:
Note: Adapted from Carsana et al. A. Early phase alveolar damage showing thick cellular debris (hyaline membrane; red arrowhead) lining an alveolus (circled in blue). B. Electron microscope image of virion-like particles inside (red arrow) and along the surface (red arrowheads) of lung cells. This article was published in Lancet Infectious Diseases, Vol 20, Carsana et al., Pulmonary post-mortem findings in a series of COVID-19 cases from northern Italy: a two-centre descriptive study, Page Nos, Copyright Elsevier 2020. This article is currently available at the Elsevier COVID-19 resource center: https://www.elsevier.com/connect/coronavirus-information-centerexternal icon.
B. Pulmonary and cardiac pathology in African American patients with COVID-19: An autopsy series from New Orleans. external iconFox et al. Lancet Respiratory Medicine (May 27, 2020).
Key findings:
- Autopsies of 10 African Americans showed extensive lung damage.
- All lungs showed diffuse alveolar damage; the extent varied and was fibrotic in one patient.
- Lungs also showed blood clots, injured blood vessels, and, in 9 patients, bleeding (Figure A&B).
- Several hearts were enlarged, probably from difficulty pumping blood through damaged lungs (Figure C).
- Heart inflammation (myocarditis) was not observed.
Methods: Autopsies of 10 African Americans (44–78 years) who died from COVID-19, New Orleans, LA. All patients had hypertension, diabetes, or obesity. Limitations: Lacked control groups without COVID-19 or without comorbidities.
Figure:
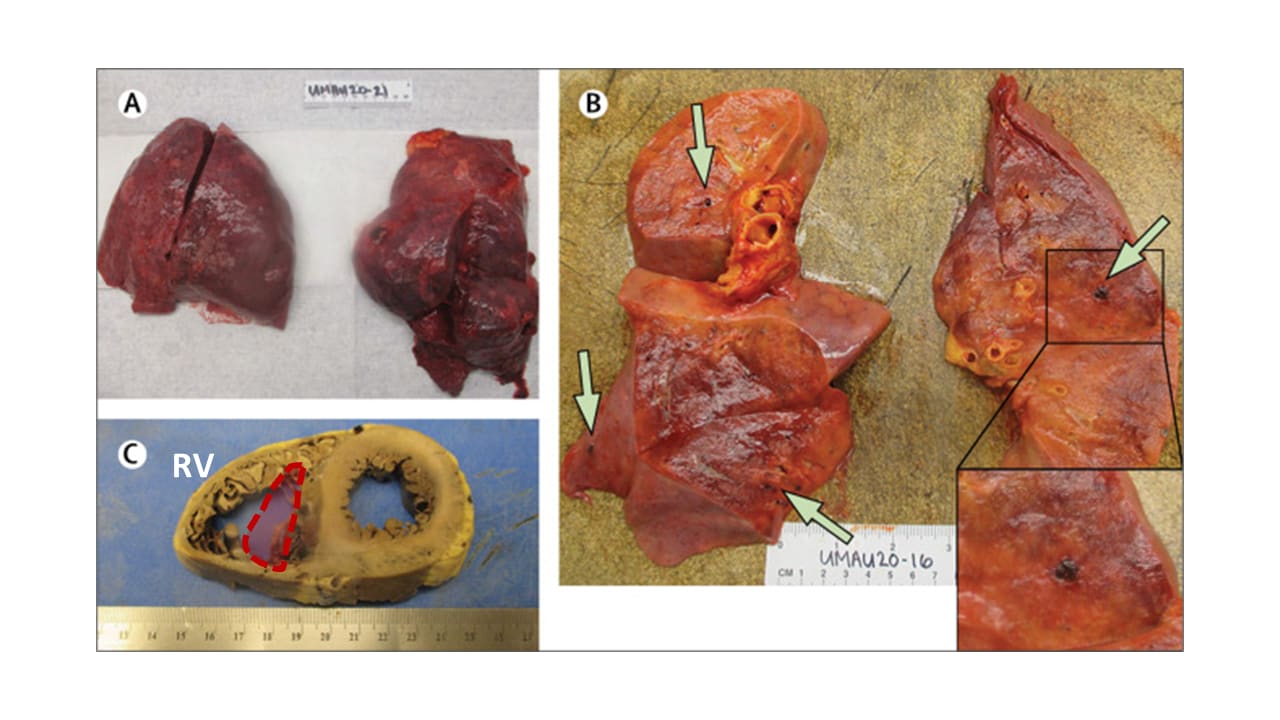
Note: Adapted from Fox et al. A. Swollen lungs with dark patches of bleeding. B. Green arrows show blood clots within small vessels. C. Cross-section of a heart with significantly dilated right ventricle (RV). Approximate normal RV size shown with red outline. This article was published in Lancet Respiratory Medicine, Vol 8, Fox et al., Pulmonary and cardiac pathology in African American patients with COVID-19: An autopsy series from New Orleans, Page 681-686, Copyright Elsevier 2020. This article is currently available at the Elsevier COVID-19 resource center: https://www.elsevier.com/connect/coronavirus-information-centerexternal icon.
C. Postmortem examination of patients with COVID-19external icon. Schaller et al JAMA (May 21, 2020).
Key findings:
- Lungs from 10 German patients showed diffuse alveolar damage (Figure).
- Destructive fibrotic scarring in one immunocompromised patient.
- Bleeding and clots were not observed.
- Mild inflammation of the heart and heart lining were seen in 4 and 2 patients, respectively, but did not meet criteria for true myocarditis.
Methods: Autopsies of 10 patients with COVID-19 (64–90 years), April 4–19, 2020, Germany. Patients had pre-existing comorbidities including hypertension (6), coronary disease (5), obesity (2), an enlarged heart (2), and emphysema (2). Limitations: Small sample size; race/ethnicity were not reported.
Figure:
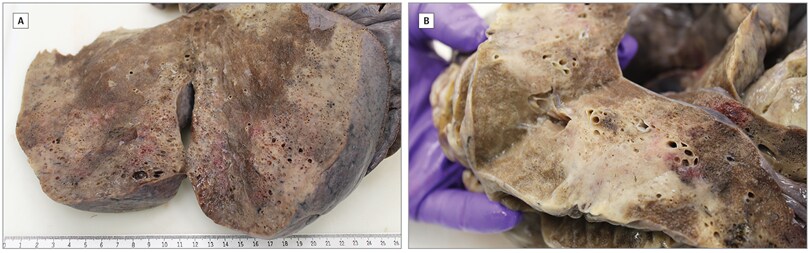
Note: Adapted from Schaller et al. End-stage diffuse alveolar damage in lungs in a patient that died from COVID-19.
Implications for 3 studies (Carsana et al., Fox et al. & Schaller et al.): As with SARS, diffuse alveolar damage was the cause of death in these COVID-19 patients. Unlike SARS, blood clots in lung blood vessels contributed to some deaths, and is consistent with prior reported observations of excess pulmonary embolism covered in Science Update 2020-05-12. Better understanding of abnormal clotting and bleeding in COVID-19 and factors that influence outcomes may inform clinical practice and the search for new treatments.
Non-Pharmaceutical Interventions
- Wise J. COVID-19: Sweden should have done more, says architect of country’s strategy.external icon BMJ. The epidemiologist responsible for Sweden’s light-touch response admits Sweden should have done more.
- Hall et al. The legal authority for states’ stay-at-home ordersexternal icon. NEJM. Discusses legal challenges to public health orders imposed during the coronavirus pandemic.
- Haushofer et al. Which interventions work best in a pandemic?external icon Science. Non-pharmaceutical interventions (NPIs) are often implemented without evidence that they work. Authors describe practical ways to use RCTs to evaluate NPIs in a pandemic setting.
Health Equity
- Curtice et al. Indigenous populations: left behind in the COVID-19 responseexternal icon. Lancet. Perspective on impact of COVID-19 on indigenous populations and ways to address the issue.
- Krishnan et al. Historical insights on coronavirus disease 2019 (COVID-19), the 1918 influenza pandemic, and racial disparities: Illuminating a path forward. external iconAnnals of Internal Medicine. Historical recount reveals how critical structural inequities and healthcare gaps contributed to and continue to compound disparate health outcomes among communities of color. Proposes 5 strategies to advance health equity.
Success Stories
- Thornton J. The “virtual wards” supporting patients with COVID-19 in the community.external icon BMJ. A Manchester hospital successfully monitors and manages COVID-19 patients at home.
- Yi-Fong Su et al. Masks and medical care: Two keys to Taiwan’s success in preventing COVID-19 spread.external icon Travel Medicine and Infectious Disease. Highlights Taiwan’s success combating SARS-CoV-2 spread in part through universal availability of surgical masks and COVID-19-related healthcare.
- Avitzur O. In Greece, COVID-19 numbers are very low. Neurologists explain why. external iconNeurology Today. Neurologists practicing in Greece describe actions taken to stave off the spread of coronavirus and speculate why they worked.
- Moris et al. Lockdown during COVID-19: The Greek success.external icon In vivo. Early lockdown contributed to the success of combating COVID-19 spread in resource-limited Greece.
Other Topics
- Cyranoski D. The biggest mystery: what it will take to trace the coronavirus source. external iconNature. Describes multiple theories of the origin of SARS-CoV-2.
- Ledford H. The coronavirus outbreak could make it quicker and easier to trial drugs.external icon Nature. Remote clinical trials and streamlined processes are a few ways that the coronavirus pandemic may permanently transform how clinical trials are conducted.
Journal Retractions
- Several high-profile retractions have brought attention to challenges posed by rapidly publishing scientific data during the coronavirus pandemic.
Disclaimer: The purpose of the CDC COVID-19 Science Update is to share public health articles with public health agencies and departments for informational and educational purposes. Materials listed in this Science Update are selected to provide awareness of relevant public health literature. A material’s inclusion and the material itself provided here in full or in part, does not necessarily represent the views of the U.S. Department of Health and Human Services or the CDC, nor does it necessarily imply endorsement of methods or findings. While much of the COVID-19 literature is open access or otherwise freely available, it is the responsibility of the third-party user to determine whether any intellectual property rights govern the use of materials in this Science Update prior to use or distribution. Findings are based on research available at the time of this publication and may be subject to change.