COVID-19 Science Update released: June 11, 2021 Edition 93

The COVID-19 Science Update summarizes new and emerging scientific data for public health professionals to meet the challenges of this fast-moving pandemic. Weekly, staff from the CDC COVID-19 Response and the CDC Library systematically review literature in the WHO COVID-19 databaseexternal icon, and select publications and preprints for public health priority topics in the CDC Science Agenda for COVID-19 and CDC COVID-19 Response Health Equity Strategy.
Section headings in the COVID-19 Science Update align with the CDC Science Agenda for COVID-19.
Section headings in the COVID-19 Science Update have been changed to align
with the CDC Science Agenda for COVID-19.
Here you can find all previous COVID-19 Science Updates.
PEER-REVIEWED
Knowing the duration of protection conferred by primary SARS-CoV-2 infection might inform better guidelines for managing contacts, but this has yet to be assessed in the general population.
A. Assessment of SARS-CoV-2 reinfection 1 year after primary infection in a population in Lombardy, Italyexternal icon. Vitale et al. JAMA Internal Medicine (May 28, 2021).
Key findings:
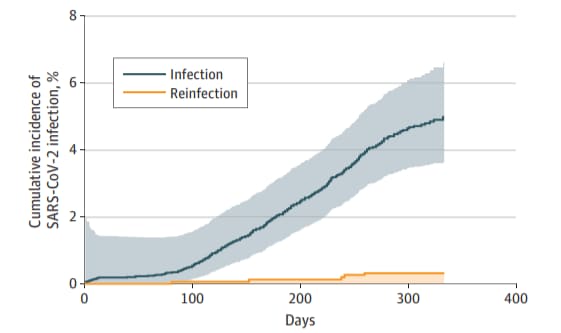
- Cumulative incidence of SARS-CoV-2 infection was significantly lower among previously infected persons compared with those with no prior infection (HR 0.06, 95% CI 0.05-0.08) (Figure).
- 0.31% (95% CI 0.03%-0.58%) were reinfected compared with 3.9% (95% CI 3.5%-4.2%) who developed primary infection.
Methods: Incidence of SARS-CoV-2 primary infection and reinfection was determined among 15,075 persons who had diagnostic SARS-CoV-2 PCR testing between February and July 2020 in Lombardy, Italy. Persons were followed until February 2021. Reinfection was defined as a second positive PCR test ≥90 days after resolution of initial infection and ≥2 consecutive negative tests between episodes. Limitations: Study was performed prior to widespread circulation of SARS-CoV-2 variants.
Figure:
Note: Adapted from Vitale et al. Cumulative incidence of SARS-CoV-2 infection among persons with no previous SARS-CoV-2 infection (n = 13,469) and those with prior infection or reinfection (n = 1,579); shaded areas represent 95% CIs. Licensed under CC BY.
B. Risk of reinfection after seroconversion to SARS-CoV-2: A population-based propensity-score matched cohort studyexternal icon. Leidi et al. Clinical Infectious Diseases (May 27, 2021).
Key findings:
- After a mean of >8 months post-serological testing, persons with antibodies to SARS-CoV-2 were less likely to develop SARS-CoV-2 infection compared with those without antibodies (HR 0.06, 95% CI 0.02-0.14).
- 1 % of seropositive persons were reinfected compared with 15.5% of seronegative persons.
Methods: Seroprevalence study conducted between April and June 2020 in Geneva, Switzerland. Persons with antibodies to SARS-CoV-2 spike protein (n = 498) were matched 1:2 to seronegative controls (n = 996) with a mean follow-up of 35 weeks to determine the risk of SARS-CoV-2 infection. Reinfection was defined by a positive PCR test and clinical investigation. Limitations: Potential for undercounting cases; study was performed prior to widespread circulation of variant viruses.
Implications for Leidi et al. and Vitale et al.: Both studies suggest that SARS-CoV-2 reinfections are rare events and that persons who have recovered from COVID-19 have minimal risk of reinfection for at least 8 months after the primary infection; the data on reinfection due to variants is still emerging.
SARS-CoV-2 variants of concern partially escape humoral but not T-cell responses in COVID-19 convalescent donors and vaccineesexternal icon. Geers et al. Science Immunology (May 25, 2021).
Key findings:
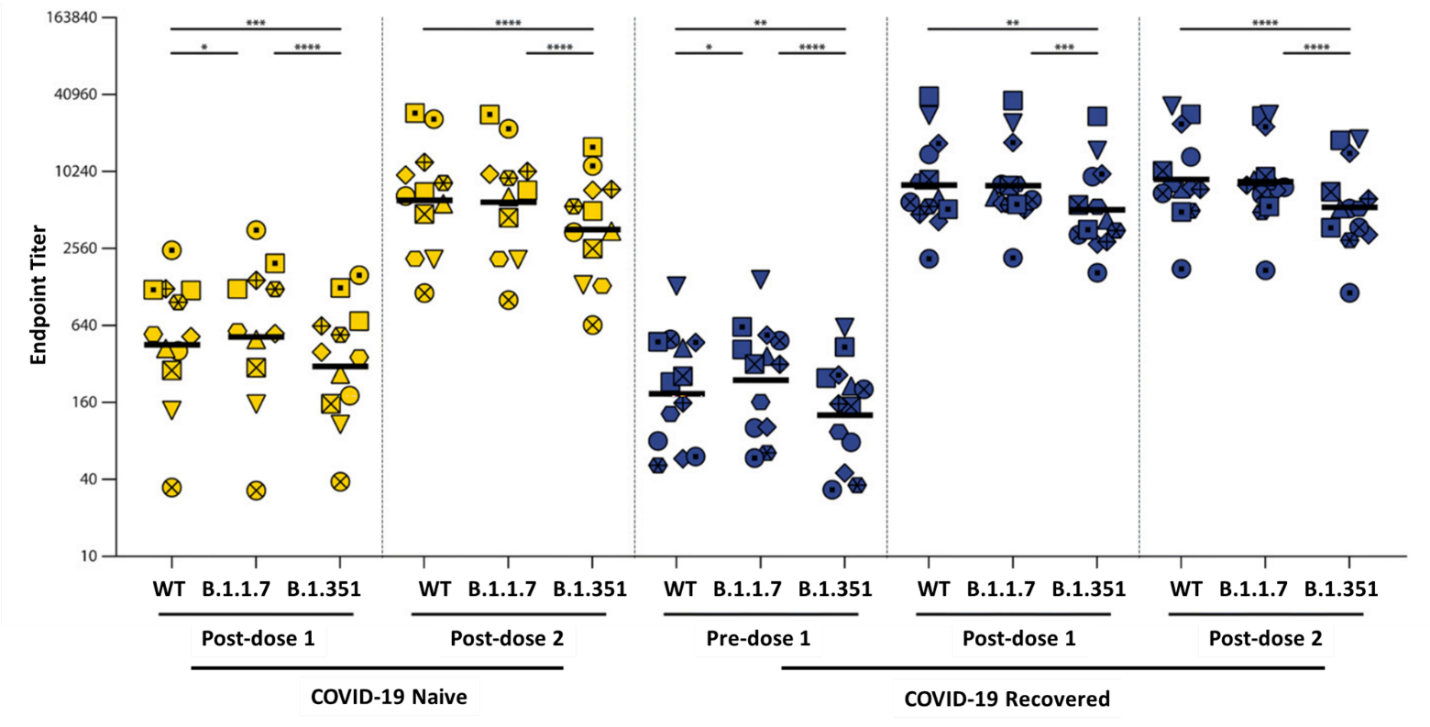
- A 2nd dose of Pfizer/BioNTech BNT162b2 vaccination:
- Was needed to boost antibody responses in SARS-CoV-2 naïve healthcare workers (HCW) (Figure).
- Did not further boost antibody responses in COVID-19-recovered participants (Figure).
- After full vaccination:
- Neutralizing antibody to B.1.351 was 2- to 4-fold lower compared to wild-type SARS-CoV-2 (Figure).
- CD4+ T-cell responses were observed in 5/7 COVID-19-naïve and 10/12 COVID-19-recovered participants.
- CD4+ T cells responded similarly to wild-type, B.1.1.7, and B.1.351 variants.
- CD8+ T cells were detected in too few participants to do further analysis.
Methods: From January 2021 onwards, healthcare workers in the Netherlands were included in a prospective vaccination study. All participants received 2 doses of the Pfizer/BioNTech BNT162b2 mRNA vaccine with a 3-week interval between doses and followed for 23 weeks post-vaccination. Antibody tests were performed on samples from 98 SARS-CoV-2 naïve participants and 23 recovered from COVID-19; 25 participants were assessed for antibody responses to variants and 19 were assessed for T-cell responses. Limitations: Small sample size; most participants were female.
Implications: Patients recovered from COVID-19 mount strong antibody responses following a single mRNA vaccine dose similar to responses among people without COVID-19 infection after two vaccine doses. While antibody responses to some circulating variants are reduced, CD4+ T-cell-mediated responses to vaccination or to previous infection do not appear to be reduced against these variants and might contribute to protection.
Figure:
Note: Adapted from Geers et al. SARS-CoV-2-specific humoral responses. Antibody binding to WT SARS-CoV-2, B.1.1.7 and B.1.351 was determined by endpoint titration in ELISA in COVID-19 naive (n = 12) and recovered (n = 13) donors before and after vaccination. Symbol shapes indicate individual donors. * p < 0.05, ** p < 0.01, *** p <0.001, **** p < 0.0001. Geers et al., May 25, 2021, SARS-CoV-2 variants of concern partially escape humoral but not T-cell responses in COVID-19 convalescent donors and vaccinees. Retrieved from https://immunology.sciencemag.org/content/6/59/eabj1750. Reprinted with permission from AAAS.
COVID-19 case investigation and contact tracing in the US, 2020.external icon Lash et al. JAMA Network Open (June 3, 2021).
Key findings:
- Of 74,185 persons having SARS-CoV-2 infection reported to health departments (HDs), 43,931 (59%) received case interviews and 24,705 (33%) provided contacts.
- Based on contact information from 6 of 8 HDs, positive test prevalence among contacts was higher than the general population (prevalence ratios [PR] 1.2-17.6).
- All 14 HDs faced challenges regarding timely collection and communication of relevant information that included insufficient personnel, unlinked databases, and incomplete data.
Methods: A cross-sectional study, over 4 weeks, that included 13 health departments and 1 Indian Health Service Unit (“health departments”) in 11 states and 1 tribal nation. Participants included all individuals with laboratory-confirmed COVID-19 and their named contacts. Trends for each study location were based on the mean weekly percentage change in incidence. Limitations: Investigators could not directly assess the effectiveness of contact tracing; effective contact tracing is dependent on transmission intensity, as well as resources available.
Implications: While contract tracing was a high-yield activity when successful, fewer than 60% of cases could be reached or named no contacts when interviewed. Therefore, contact tracing was unlikely to have had a major impact on SARS-CoV-2 transmission.
PEER-REVIEWED
Neutralising antibody activity against SARS-CoV-2 VOCs B.1.617.2 and B.1.351 by BNT162b2 vaccination. external iconWall et al. Lancet (June 3, 2021).
Key findings:
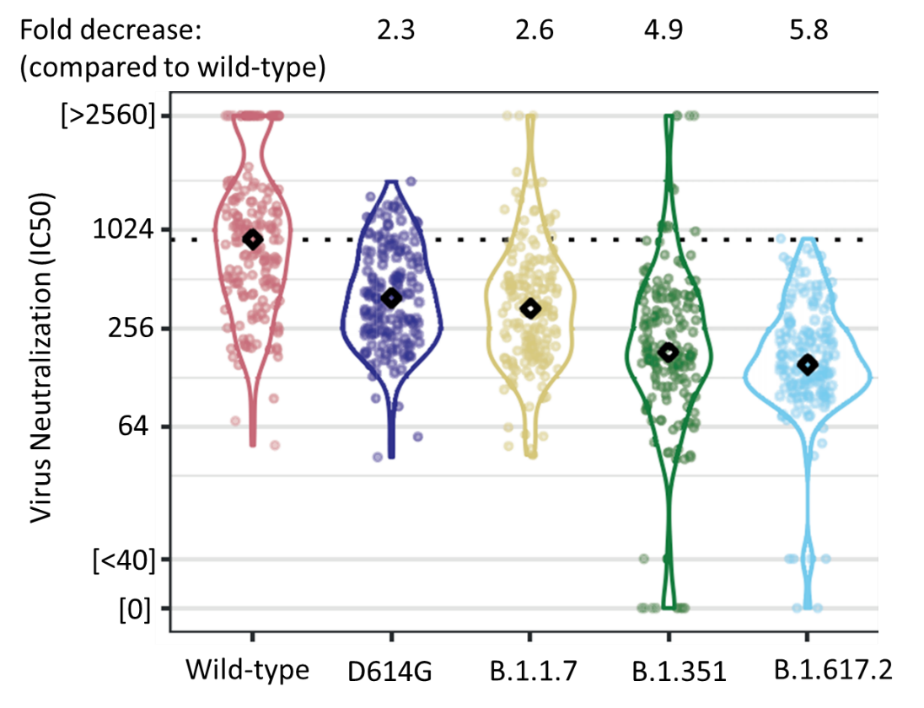
- 2 doses of the Pfizer/BioNTech BNT162b2 vaccine elicited lower neutralizing antibody titers (NAbT) against SARS-CoV-2 variants compared to wild-type SARS-CoV-2 (Figure), showing a:
- 2.3-fold (95% CI 1.9-2.6) decrease against D614G.
- 2.6-fold (95% CI 2.2-3.1) decrease against B.1.1.7.
- 4.9-fold (95% CI 4.2-5.7) decrease against B.1.351.
- 5.8-fold (95% CI 5.0-6.9) decrease against B.1.617.2.
- Older age correlated with reduced NAbT against wild-type SARS-CoV-2 and all variants.
- Compared with 2 doses, NAbTs following 1 dose were significantly lower, and median NAbT against B.1.351 and B.1.617.2 were below the quantitative limit of detection.
Methods: Serum samples from a SARS-CoV-2 longitudinal study among UK healthcare workers (n = 250) in January 2021 were tested for neutralization against wild-type and four variants: D614G, B.1.1.7, B.1.351, and B.1.617.2. Neutralization was compared after either 1 dose (n = 149) or 2 doses (n = 159) of Pfizer/BioNTech BNT162b2. Limitations: Small sample size and single-ethnicity participants.
Implications: These findings suggest reduced vaccine efficacy against B.1.351 and B.1.617.2, particularly after 1 dose and in older adults. Vaccination strategies that promote 2-dose protection should be prioritized.
Figure:
Note: Neutralizing antibody titers (NAbTs) after 2 doses of Pfizer/BioNTech BNT162b2 vaccine for wild-type and 4 variants (D614G, B.1.1.7, B.1.351, and B.1.617.2). Horizontal dotted line displays median IC50 for wild-type strain as reference. Permission request in process.
Symptomatic acute myocarditis in seven adolescents following Pfizer-BioNTech COVID- 19 vaccinationexternal icon. Marshall et al. Pediatrics (June 1, 2021).
Key findings:
- 7 US-based adolescent males developed myocarditis or myopericarditis within 4 days of receiving the 2nd dose of Pfizer/BioNTech BNT162b2.
- None had evidence of acute SARS-CoV-2 infection and 6 had negative SARS-CoV-2 antibody tests, suggesting no prior infection.
- None met criteria for multi-system inflammatory syndrome in children.
- All diagnostic evaluations for myocarditis etiologies were negative.
- All cases were mild and responded quickly to minimally invasive therapy.
Methods: Case series of clinical myocarditis and myopericarditis in 14- to 19-year-old males after receipt of 2nd dose of Pfizer/BioNTech BNT162b2 vaccine. All patients were vaccinated in April or May 2021. Myocarditis was diagnosed based on cardiac MRI. Limitations: Case identification was not systematic; not possible to exclude all alternative etiologies.
Implications: Post-vaccination myocarditis, as seen here and in a similar case series by Snapiri et alexternal icon, should be reported to national surveillance systems such as the Vaccine Adverse Events Reporting System, which would allow prompt investigation of sporadic clinical reports. The authors and an accompanying editorial by O’Leary and Maldonadoexternal icon emphasize that the benefits of vaccination against highly transmissible and potentially fatal SARS-CoV-2 infection clearly outweigh risks.
PREPRINTS (NOT PEER-REVIEWED)
Emerging SARS-CoV-2 variants of concern evade humoral immune responses from infection and vaccinationexternal icon. Caniels et al. medRxiv (June 1, 2021). Published in Science Advancesexternal icon (September 3, 2021).
Key findings:
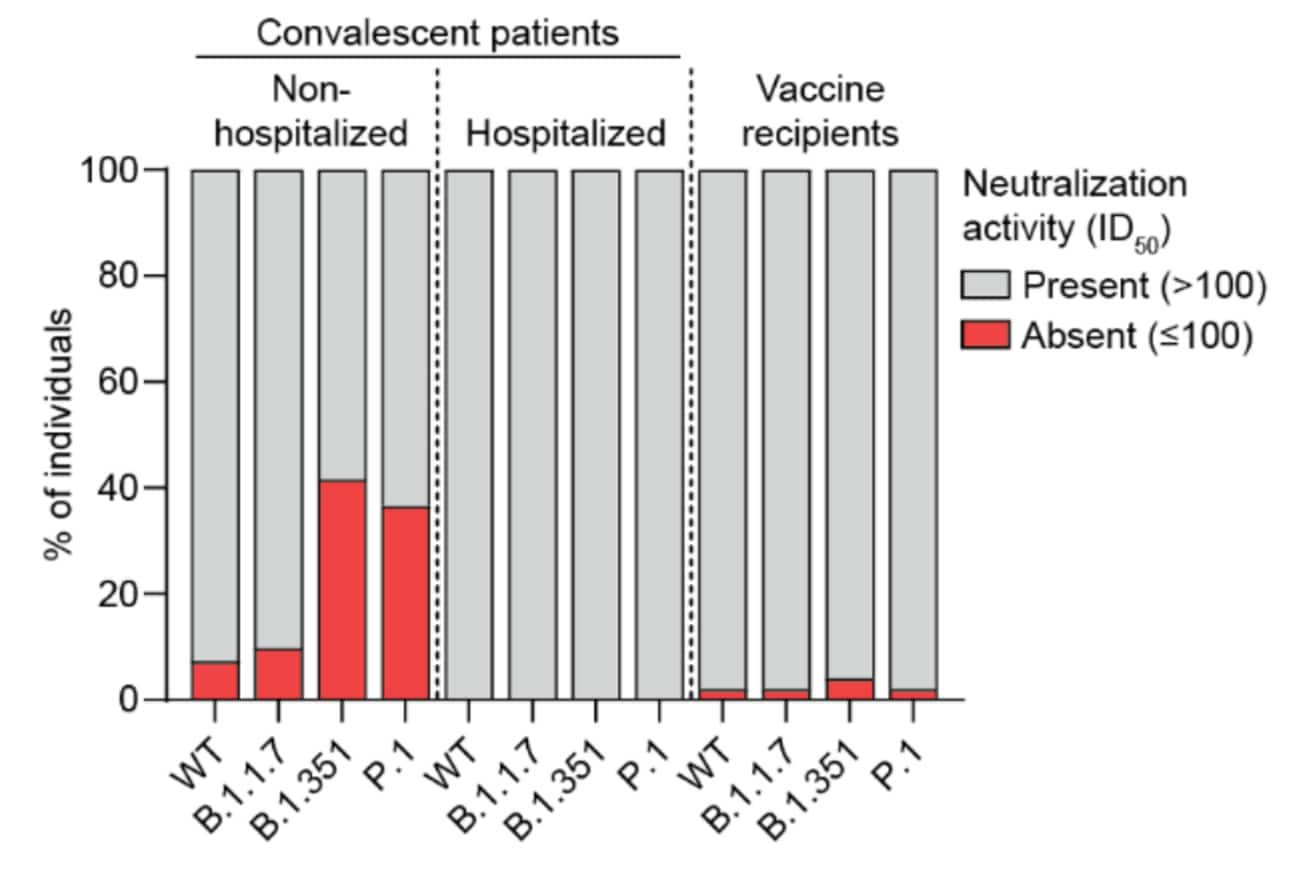
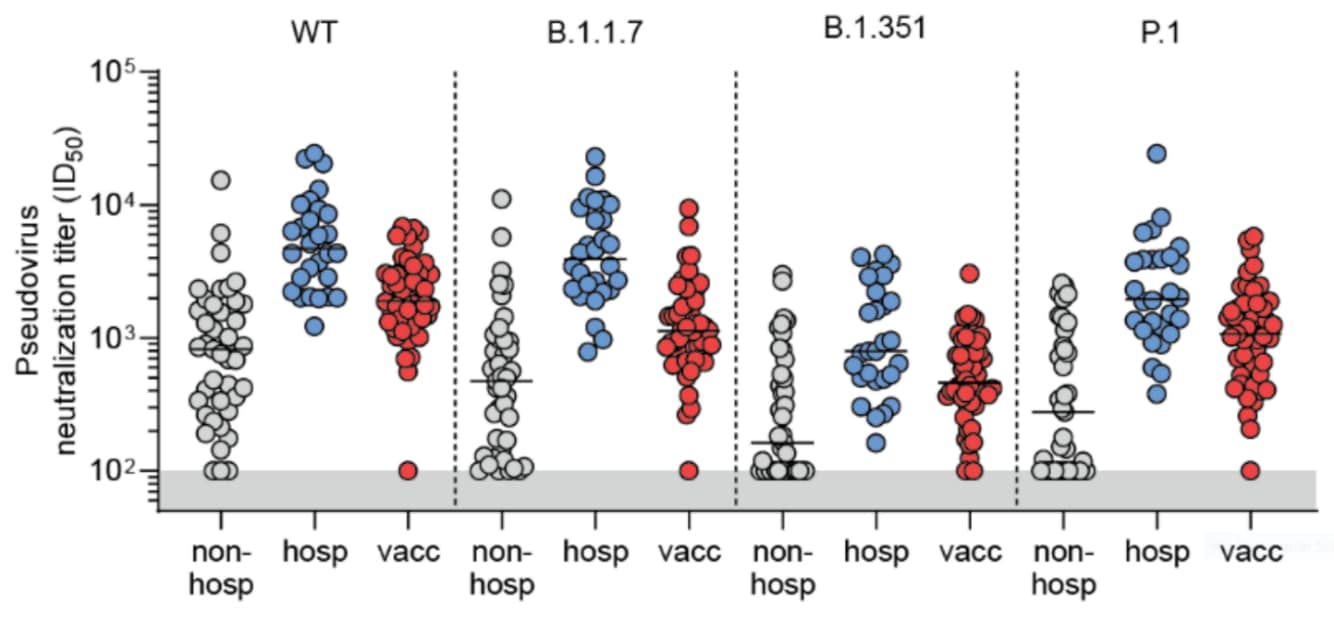
- Hospitalized convalescent COVID-19 patients and recipients of Pfizer/BioNTech BNT162b2 vaccine produced sufficient antibody titers to neutralize pseudoviruses of B.1.1.7, B.1.351, and P.1 (Figure 1).
- Among unvaccinated, non-hospitalized convalescent cases, sera from 39% (16/41) were unable to neutralize B.1.351 and 34% (14/41) were unable to neutralize P.1 (Figure 1).
- Neutralizing antibody titers were lower in persons with mild SARS-CoV-2 infection compared with other groups, particularly against B.1.351 and P.1 (Figure 2).
Methods: A cohort study measuring neutralizing antibodies in sera from 69 SARS-CoV-2 infected adults 4–6 weeks after symptom onset (41 with mild infection, 28 hospitalized with COVID-19) and 50 health care workers 4 weeks after second dose of Pfizer/BioNTech BNT162b2, in the Netherlands, between March 2020 and January 2021. Limitations: Only 8% of participants over age 60; did not examine immune effector functions other than virus neutralization.
Implications: Persons with mild SARS-CoV-2 infection might not develop robust neutralizing antibody responses or protective immunity against all strains of infection, underscoring the importance of vaccination even in previously infected individuals.
Figure 1:
Note: Adapted from Caniels et al. Presence or absence of neutralizing antibodies against SARS-CoV-2 pseudovirus expressing the spike protein from wild-type (WT) SARS-CoV-2 and 3 variants in sera from non-hospitalized persons infected with SARS-CoV-2, hospitalized COVID-19 patients, or fully vaccinated persons. ID50: half-maximal neutralizing dilution. Used by permission of authors.
Figure 2:
Note: Adapted from Caniels et al. Neutralizing antibody titers against SARS-CoV-2 pseudovirus expressing the spike protein from wild-type (WT) SARS-CoV-2 and 3 variants in sera from unvaccinated SARS-CoV-2-positive persons who did not require hospitalization (non-hosp, n = 41) and hospitalized COVID-19 patients (hosp, n = 28), and fully vaccinated participants (vacc, n = 50). Gray shaded area represents negative values. ID50: half-maximal inhibitory dilution. Used by permission of authors.
The indirect effect of mRNA-based COVID-19 vaccination on unvaccinated household members.external icon Salo et al. medRxiv (May 29, 2021).
Key findings:
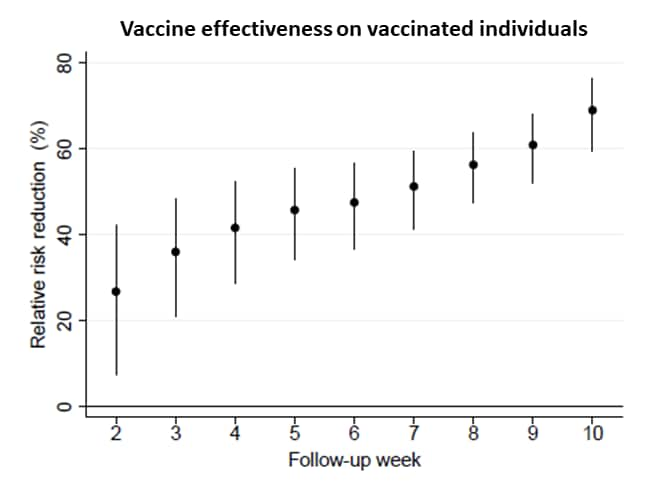
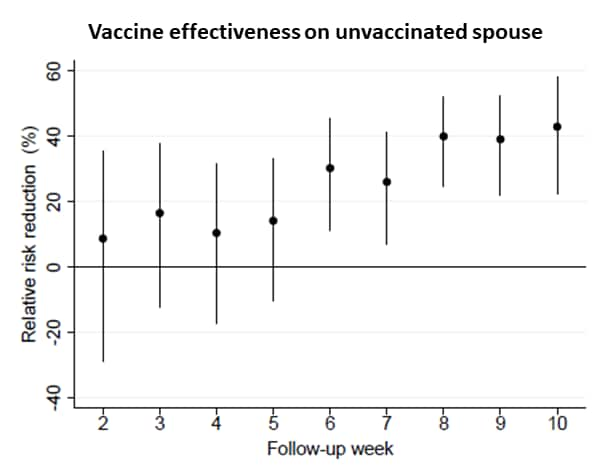
- After 1 dose of mRNA vaccine, effectiveness increased over time for both vaccinated individuals and their unvaccinated spouses (Figure).
- Among vaccinated individuals, the relative risk reduction was 26.8% (95% CI 7.5%-42.1%) 2 weeks later and 69.0% (95% CI 59.2%-76.3%) 10 weeks later.
- Among unvaccinated spouses, the relative risk reduction was 8.7% (95% CI -28.9%-35.4%) 2 weeks later and 42.9% (95% CI 22.3%-58.1%) 10 weeks later.
Methods: Data from a mass vaccination program in Finland between December 2020 and March 24, 2021 looking at the effectiveness of mRNA-based COVID-19 vaccines in reducing the cumulative risk of infection among vaccinated healthcare workers (n = 95,138) and their unvaccinated spouses (n = 52,766). Effectiveness estimates were reported by follow-up week after receiving the first dose. Limitations: The extent of contact of spouses with SARS-CoV-2-infected individuals is unclear.
Implications: mRNA-based vaccines not only prevent SARS-CoV-2 infections among vaccinated individuals but lead to a substantial reduction in infections among unvaccinated household members.
Figure:
Note: Adapted from Salo et al. Vaccine effectiveness in reducing risk for vaccinated individuals (top panel) and their unvaccinated spouses (bottom panel). Time (x-axis) is weeks after first dose of mRNA-based vaccine. Relative risk reduction (y-axis) was calculated using a log-binomial regression model. Error bars represent 95% CIs. Licensed under CC-BY-NC-ND 4.0.
Genomic characterization and epidemiology of an emerging SARS-CoV-2 variant in Delhi, India.external icon Dhar et al. medRxiv (June 3, 2021). Published in Scienceexternal icon (October 14, 2021).
Key findings:
- COVID-19 cases exponentially increased in India in April 2021, with expansion of highly transmissible B.1.617 variants, compared to earlier in the pandemic.
- Despite high case burden, case fatality rate was significantly reduced during this time frame.
- Of 27 breakthrough infections following vaccination, 19 were B.1.617.2, 6 were B.1, and 2 were B.1.617.1
Methods: Records of all tests performed, positive cases, positivity rate and deaths, were accessed through the Integrated Disease Surveillance Program at the National CDC (NCDC) of India. Sequence data from >9000 samples were used to characterize the epidemiology of sequential SARS-CoV-2 waves in Delhi between November 2020 and May 2021. Samples from 27 vaccination breakthrough cases at NCDC were sequenced. Limitations: Unable to review data from all COVID-related deaths during the study period.
Implications: Findings suggest that the recent wave of COVID-19 in India was largely due to emergence of a more transmissible variant, B.1.617.2.
Heterologous ChAdOx1 nCoV-19 and BNT162b2 prime-boost vaccination elicits potent neutralizing antibody responses and T cell reactivity.external iconGroß et al. medRxiv (June 1, 2021). Published in eBioMedicine as Heterologous ChAdOx1 nCoV-19 and BNT162b2 prime-boost vaccination elicits potent neutralizing antibody responses and T cell reactivity against prevalent SARS-CoV-2 variantsexternal icon (December 17, 2021).
Key findings:
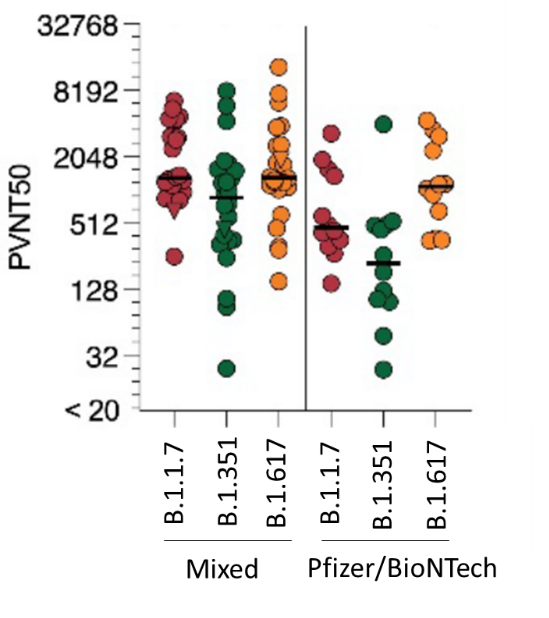
- Compared with participants receiving 2 doses of the same vaccine, participants receiving a mixed vaccination regimen had higher neutralizing antibody titers against B.1.1.7, B.1.351, and B.1.617 variants (Figure).
- 19/19 participants had CD4+ T-cell responses and 17/19 had CD8+ T-cell responses 2 weeks after mixed prime-boost vaccination.
Methods: A mixed COVID-19 vaccine schedule comprised of Oxford/AstraZeneca ChAdOx1 (dose 1 = prime) followed 8 weeks later with Pfizer/BioNTech BNT162b2 (dose 2 = boost) was compared to two doses of Pfizer/BioNTech in 26 adults aged 25–46 years in Germany. B cell responses were measure in all participants and T cell responses in a subset of 19 participants. Limitations: Small sample size; limited to younger adults; did not compare different vaccination intervals.
Implications: Among healthy young adults, heterologous prime-boost vaccination resulted in high immunogenicity. These findings suggest a role for interchanging adenoviral and mRNA vaccines in areas of vaccine shortage.
Figure:
Note: Adapted from Groß et al. Half-maximal virus neutralization titers (PVNT50) against pseudovirus expressing spike protein from B.1.1.7, B.1.351, and B.1.617 variants with a mixed vaccine or 2 doses of the same vaccine, 14 days after 2nd dose (B). Bars represent median PVNT50 values per group. Used by permission of author.
Detection, Burden, and Impact
- Phetsouphanh et al. Immunological dysfunction persists for 8 months following initial mild-moderate SARS-CoV-2 infection.external icon medRxiv (Preprint; June 3, 2021).Published in Nature Immunologyexternal icon (January 13, 2022). A prospective cohort of 31 persons in Australia with long COVID following asymptomatic SARS-CoV-2 infection showed elevated serum levels of 15 proinflammatory mediators (cytokines and chemokines) that were not observed in COVID-19 survivors without long COVID or persons recovered from other coronavirus or viral infections.
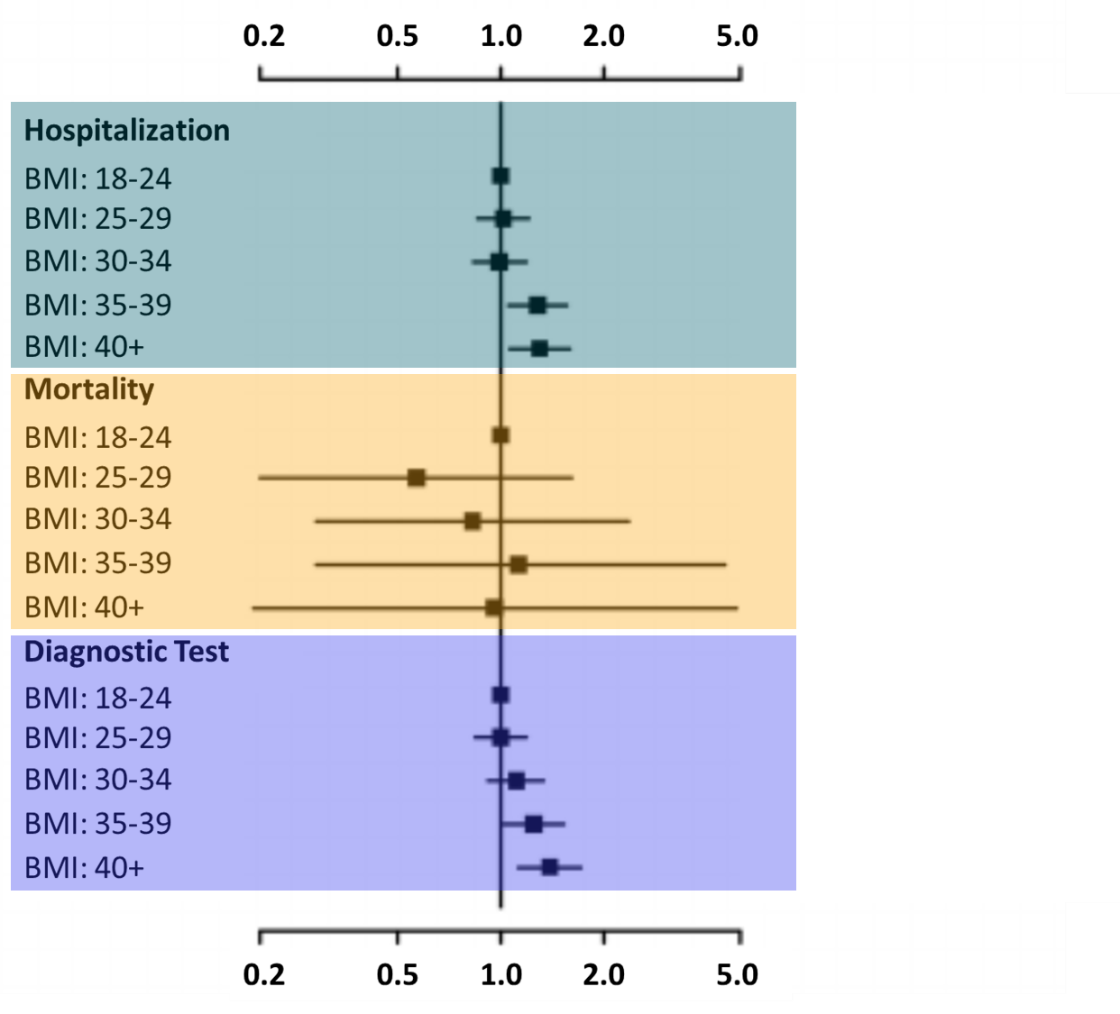
- Aminian et al. Association of obesity with post-acute sequelae of COVID-19 (PASC).external icon Diabetes, Obesity and Metabolism (June 1, 2021). Among 2,839 SARS-CoV-2 patients that did not initially require hospitalization, patients with body mass index (BMI) >40 (HR 1.30, 95% CI 1.06-1.59 ) or BMI 30–35 (HR 1.28, 95% CI 1.05-1.56) had increased risk of hospitalization compared with patients with a normal BMI (18–24), at a median of 8 months after a positive test.
Note: Adapted from Aminian et al. Hazard ratios (HR) for risk of hospitalization, mortality, and additional diagnostic testing by body mass index (BMI) 8 months after a positive SARS-CoV-2 test. Used with permission. © John Wiley & Sons Ltd.
- Hag-Ali et al. The detection dogs test is more sensitive than real-time PCR in screening for SARS-CoV-2external icon. Communications Biology (June 3, 2021). Dogs trained to identify SARS-CoV-2-infected individuals using olfactory signals correctly identified 3,246 of 3,272 PCR-negative persons (specificity = 99.2%) and 15 of 18 PCR-positive persons (sensitivity = 83.3%) among a group of 3,290 randomly selected adult males in Abu Dhabi.
Note: Adapted from Hag-Ali, et al. Trained sniffer dogs and trainers worked as teams. An olfactory test apparatus is visible in the background. Licensed under CC BY 4.0.
Prevention, Mitigation, and Intervention Strategies
- Takuva et al. Thromboembolic events in the South African Ad26.COV2.S vaccine study.external icon NEJM (June 2, 2021). Interim safety data between February 17 and April 12, 2021, from 288,368 health care workers reported only 5 thromboembolic events in participants receiving the Janssen (Johnson & Johnson) AD26.COV2.S vaccine, or 1.7 per 100,000 participants. All were in women, 4 of whom had risk factors for thromboembolism; there was 1 fatality.
- Patel et al. Association of simulated COVID-19 vaccination and nonpharmaceutical interventions with infections, hospitalizations, and mortalityexternal icon. JAMA Network Open (June 1, 2021). A decision analytical model incorporating social inequities found that stopping NPIs during vaccine distribution substantially increased infections, hospitalizations, and deaths. As NPIs were removed, higher coverage with less effective vaccines would reduce risk more than lower coverage with more effective vaccines.
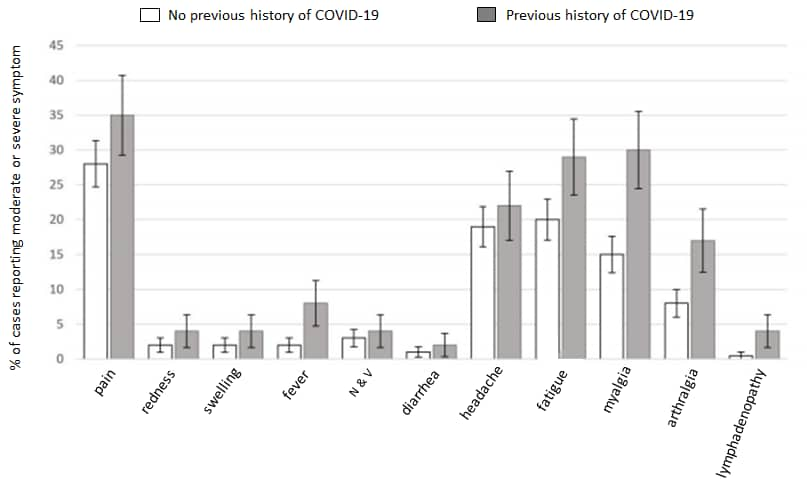
- Raw et al. Previous COVID-19 infection, but not long-COVID, is associated with increased adverse events following BNT162b2/Pfizer vaccinationexternal icon. Journal of Infection (May 29, 2021). The percentage of healthcare workers (n = 974) reporting moderate and severe symptoms after the first dose of Pfizer/BioNTech BNT126b2 vaccine was greater among participants with a previous history of COVID-19 compared with participants with no history of COVID-19 (56% v 47%, OR: 1.5 [95% CI 1.1-2.0]). Symptoms following vaccination were not more likely in individuals with, compared to without, long COVID.
Note: Adapted from Raw, et al. Percentage of cases reporting various symptoms by COVID-19 status. Symptom onset was mostly within 24 hours (75%) with no onset >48 hours. Reprinted from Journal of Infection, online May 29, 2021, Raw et al. Previous COVID-19 infection, but not long-COVID, is associated with increased adverse events following BNT162b2/Pfizer vaccination. Copyright 2021, with permission from Elsevier.
- Ku et al. Nasal delivery of an IgM offers broad protection from SARS-CoV-2 variants.external icon Nature (June 3, 2021). Using a mouse model of SARS-CoV-2, an IgM antibody (IgM-14) engineered for intranasal administration potently neutralized B.1.1.7, P.1, B.1.351, and 21 other variant RBDs, many resistant to IgG monoclonal antibodies authorized for emergency use. IgM-14 was >230-fold more potent than its parental IgG-14 in neutralizing B.1.1.7.
Social, Behavioral, and Communication Science
- Teasdale et al. COVID-19 testing among children, parental preferences for testing venues and acceptability of school-based testing: a survey of US parentsexternal icon. medRxiv (Preprint; May 29, 2021). Published in Public Health Reports as COVID-19 testing among US children, parental preferences for testing venues, and acceptability of school-based testingexternal icon (January 13, 2022). In a national online survey of 2,074 US parents conducted in March 2021, 50.6% of parents said they would allow their youngest child to be tested for SARS-CoV-2 at school or daycare if required; 33.5% said they would not allow school-based testing. The preferred testing venue was pediatrician’s office.
Disclaimer: The purpose of the CDC COVID-19 Science Update is to share public health articles with public health agencies and departments for informational and educational purposes. Materials listed in this Science Update are selected to provide awareness of relevant public health literature. A material’s inclusion and the material itself provided here in full or in part, does not necessarily represent the views of the U.S. Department of Health and Human Services or the CDC, nor does it necessarily imply endorsement of methods or findings. While much of the COVID-19 literature is open access or otherwise freely available, it is the responsibility of the third-party user to determine whether any intellectual property rights govern the use of materials in this Science Update prior to use or distribution. Findings are based on research available at the time of this publication and may be subject to change.