COVID-19 Science Update released: May 19, 2020 Edition 14

The COVID-19 Science Update summarizes new and emerging scientific data for public health professionals to meet the challenges of this fast-moving pandemic. Weekly, staff from the CDC COVID-19 Response and the CDC Library systematically review literature in the WHO COVID-19 databaseexternal icon, and select publications and preprints for public health priority topics in the CDC Science Agenda for COVID-19 and CDC COVID-19 Response Health Equity Strategy.
Section headings in the COVID-19 Science Update have been changed to align
with the CDC Science Agenda for COVID-19.
Here you can find all previous COVID-19 Science Updates.
PEER-REVIEWED
Clinical characteristics and results of semen tests among men with coronavirus disease 2019external icon. Li et al. JAMA Network Open (May 7, 2020; Correctionexternal icon on June 1, 2020).
Key findings:
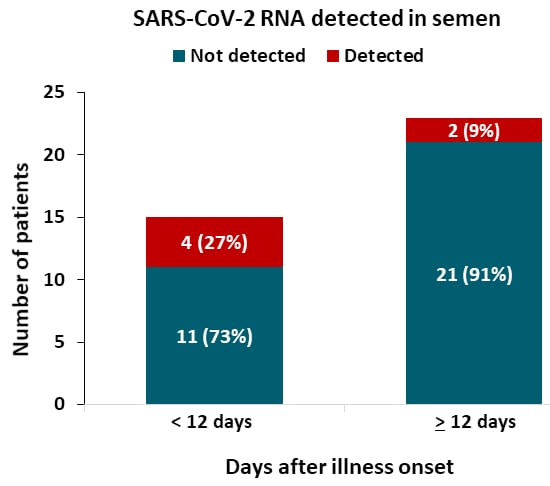
- Semen samples from 6 of 38 patients (15.8%) were positive for SARS-CoV-2 RNA.
- 4 of 15 (27%) were collected <12 days after onset (Figure).
- 2 of 23 (9%) were collected ≥12 days after onset.
Methods: 38 males age >15 years hospitalized with COVID-19 at a single hospital, Shangqiu, China. SARS-CoV-2 RNA RT-PCR testing of semen samples. Limitations: Small sample size; no repeat testing to determine duration of viral RNA in semen; unknown if viral RNA detected in semen reflects presence of live virus.
Implications: This is the first report of SARS CoV-2 RNA detection in semen, but this report does not establish that the virus can be sexually transmitted. Additional research is needed to assess whether infectious SARS-CoV-2 can occur in semen.
Figure:
Note: Adapted from Li et al. SARS CoV-2 RNA detected or not detected by PCR in semen obtained from patients < 12 days or ≥ 12 days after illness onset.
Note: Adapted from Li et al. SARS CoV-2 RNA detected or not detected by PCR in semen obtained from patients < 12 days or ≥ 12 days after illness onset. Licensed under CC-BY.
SARS-CoV-2 rates in BCG-vaccinated and unvaccinated young adultsexternal icon. Hamiel et al. JAMA (May 13, 2020).
Key findings:
- Rates of COVID-19 did not differ between cohorts of persons routinely vaccinated with BCG and those not vaccinated with BCG.
Methods: Cohort study comparing SARS-CoV-2 RNA RT-PCR-positive test results among individuals born during the 3-year time periods before and after cessation of Israel’s universal newborn BCG immunization program in 1982 (1979-1981 [N = 3,064] and 1983-1985 [N = 2,869], respectively). PCR results obtained for people with COVID-19 symptoms were tested during March 1 and April 5, 2020. The proportions and rates per 100,000 people of positive test results in the 2 groups (cohorts routinely vaccinated with BCG vs. not vaccinated with BCG) were compared using chi square tests. Limitations: Ecological study; individual BCG vaccination status was not assessed; only symptomatic individuals were tested for COVID-19.
Implications: In this study, BCG vaccination did not appear to provide protection against SARS-CoV-2 infection.
COVID-19 in children is milder than in adults, and severe illness is rare in infants and children. However, children can become critically ill with COVID-19. Some features of illness in children mirror those seen in adults, but there is emerging data that there are also features that are unique to infants and children.
PEER-REVIEWED
A. Characteristics and outcomes of children with coronavirus disease 2019 (COVID-19) infection admitted to US and Canadian pediatric intensive care unitsexternal icon. Shekerdemian et al. JAMA Pediatrics (May 11, 2020).
Key findings:
- Among 48 children with COVID-19 admitted to ICUs, 35 (73%) presented with respiratory symptoms, 33 (69%) had severe or critical illness, 18 (38%) required mechanical ventilation, 11 (23%) had failure of two or more organ systems, and 2 (4%) died.
- 40 (83%) had pre-existing comorbidities; 19 (40%) had developmental or genetic disorders with long-term dependence on technological support (including tracheostomy). Other comorbidities included cancer, diabetes, obesity, and seizures.
Methods: Retrospective review of 48 patients (25 male and 23 female) aged ≤16 with confirmed COVID-19 in pediatric ICUs in 14 US hospitals, between March 14 and April 3, 2020. Limitations: 15 (31%) patients remained hospitalized at the time of the report; small sample size.
B. COVID-19 in children with cancer in New York Cityexternal icon. Boulad et al. JAMA Oncology (May 13, 2020).
Key findings:
- Among 20 children who tested positive for SARS-CoV-2 infection at a pediatric cancer hospital, only one required hospital admission for COVID-19 but did not need ICU-level care.
Implications of both studies (Shekerdemian et al. & Boulad et al.): Most children with symptomatic COVID-19 have pre-existing comorbidities. However, severe COVID-19 among pediatric cancer patients may be low.
The public health and clinical resources required to respond to the COVID-19 pandemic are considerable. Diversion of these resources to address this unprecedented event as well, as recommendations for social distancing and public concern about exposure to SARS-CoV-2 in health care settings, have likely contributed to lapsed or delayed care of non-COVID-19-related medical issues.
PEER-REVIEWED
Delayed access or provision of care in Italy resulting from fear of COVID-19external icon. Lazzerini et al. Lancet Child & Adolescent Health (April 9, 2020).
Key findings:
- Visits to 5 pediatric emergency departments decreased 77% during March 2020 compared with the same period in 2018 and 2019 (Figure).
- 12 children with delayed care had serious conditions at the time of hospitalization, including diabetic ketoacidosis, acute leukemia, prolonged seizures, sepsis, hypovolemic shock, hypoglycemia, abdominal tumor, and complications of cerebral palsy.
- 6 were admitted to ICUs and 4 died.
- Among those with serious conditions, parents reported delays in seeking care because of fear of COVID-19. In 5 (42%) cases, a health provider was contacted, but unavailable or hospital access was discouraged.
Methods: Retrospective analysis of hospital data on visits to 5 pediatric emergency departments in Italy between March 1 and 27, 2020 compared with visits during the same period in 2018 and 2019. Cases of delayed care were reviewed. Limitations: Delayed care not assessed systematically; school and sports cancellation may have contributed to fewer infections and injuries requiring care.
Figure:
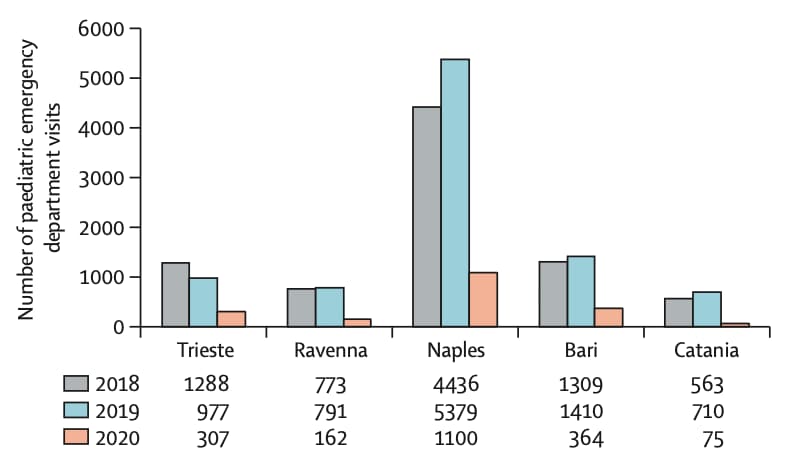
Note: Adapted from Lazzerini et al. Visits to pediatric emergency departments in 5 Italian cities during March 1–27, 2020 compared with the same period in 2018 and 2019. This article was published in Lancet Child & Adolescent Health, Vol 4, Lazzerini et al., Delayed access or provision of care in Italy resulting from fear of COVID-19, Page E10-E11, Copyright Elsevier 2020. This article is currently available at the Elsevier COVID-19 resource center: https://www.elsevier.com/connect/coronavirus-information-center.
B. Collateral effect of COVID-19 on stroke evaluation in the United Statesexternal icon. Kansagra et al. NEJM (May 8, 2020).
Key findings:
- The number of patients evaluated for suspected acute stroke in assessed US hospitals decreased by 39% early in the COVID-19 pandemic (Figure).
- Decreases were seen across all age, sex, and stroke severity subgroups.
Methods: Retrospective analysis of a commercial database of 231,753 patients who underwent neuroimaging for acute ischemic stroke at 856 US hospitals between July 2019 and April 27, 2020. Daily patient counts during a 29-day pre-pandemic period (February 1-29, 2020) were compared with those in a 14-day period during the early outbreak (March 26- April 8, 2020). Limitations: Neuroimaging was a surrogate for care provided; findings may not be generalizable.
Figure:
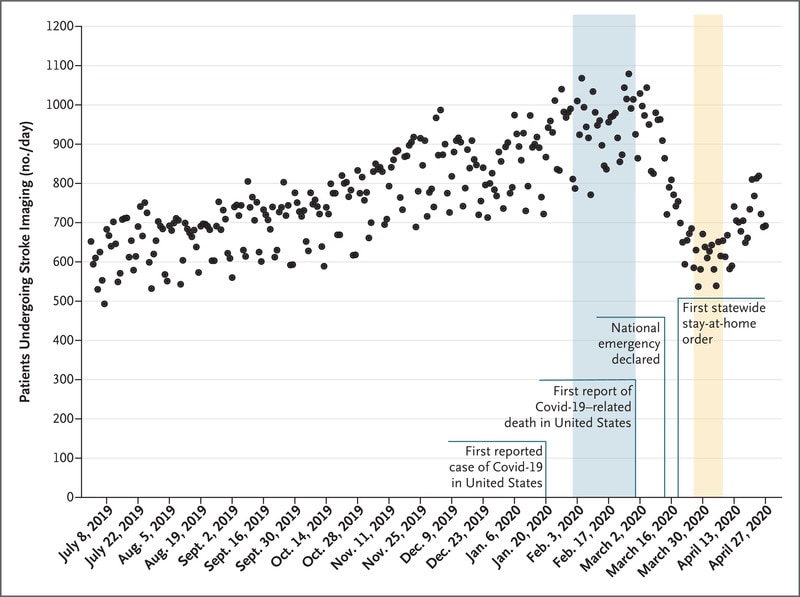
Note: Adapted from Kansagra et al. Daily counts of patients who underwent neuroimaging for stroke in the United States, during the pre-pandemic era (February 1—29, 2020) and early-pandemic era (March 26 – April 8, 2020). From NEJM. 383:400-401. DOI: 10.1056/NEJMc2014816Copyright ©2020 Massachusetts Medical Society. Reprinted with permission from Massachusetts Medical Society.
C. Organ procurement and transplantation during the COVID-19 pandemicexternal icon. Loupy et al. Lancet (May 11, 2020).
Key findings:
- Cadaver organ transplants decreased 91% in France and 51% in the US (Figure) during the COVID-19
- Reduction driven by decreases in kidney transplants.
Methods: Retrospective study of daily transplantation counts by organ type collected from French and American national registries, between early March and mid-April 2020. Limitations: Unclear what dates were compared to calculate the percentage decrease; no transplant counts pre-COVID for comparison.
Figure:
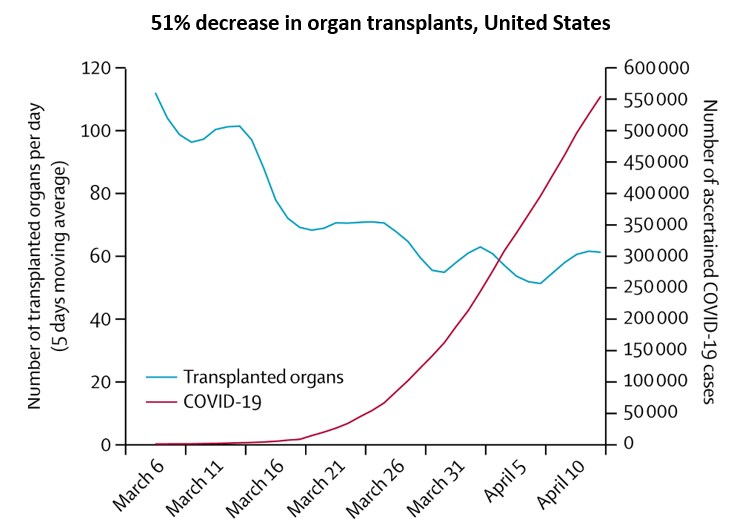
Note: Adapted from Loupy et al. Number of organ transplants from deceased donors per day and cumulative COVID-19 cases in the United States, early March – mid-April 2020. This article was published in Lancet, Vol 395, Loupy et al., Organ procurement and transplantation during the COVID-19 pandemic, Page E95-E96, Copyright Elsevier 2020. This article is currently available at the Elsevier COVID-19 resource center: https://www.elsevier.com/connect/coronavirus-information-center.
Implications of 3 studies (Lazzerini et al., Kansagra et al., & Loupy et al.): The COVID-19 pandemic has adversely impacted the timely receipt of urgently needed, non-COVID-19-related medical care, including stroke management, organ transplantation, and pediatric emergency care.
Prolonged persistence of SARS-CoV-2 RNA in body fluids. Sun et al. Emerging Infectious Diseases (May 8, 2020).
Key findings:
- Average time from onset of COVID-19 symptoms to negative nasopharyngeal PCR was > 3 weeks, and longer in severe than mild cases (Figure).
- Severe cases: median, 5 days; 95th percentile, 49.4 days.
- Mild cases: median, 7 days; 95th percentile, 46.3 days.
Methods: SARS-CoV-2 RT-PCR testing of throat, nasopharynx, sputum, and feces of 49 hospitalized COVID-19 patients (43 mild and 6 severe cases). Estimated time from symptom onset to first negative result after the final positive result (median and 95th percentile) using parametric Weibull regression. Limitations: Small sample size; 67% of specimens not collected systematically; no viral culture results.
Implications: Viral shedding can be prolonged in hospitalized COVID-19 patients. Additional studies are needed to determine infectivity of viral particles after clinical recovery.
Figure:
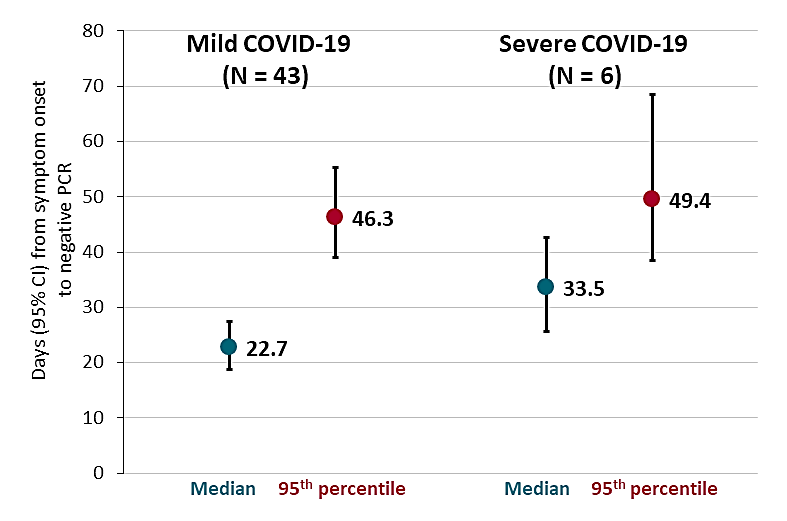
Note: Adapted from Sun et al. Days (95% CIs) from onset of COVID 19 symptoms to negative nasopharyngeal RT-PCR (median and 95th percentile) among mild (N = 43) and severe (N = 6) cases. Open access journal; all content freely available.
Vaccine Development
- Shah et al. Ethics of controlled human infection to study COVID-19external icon. Controlled human infection (CHI) studies, where a small number of participants are deliberately exposed, have been proposed to accelerate SARS-CoV-2 vaccine development. An ethical framework for research sponsors, communities, participants, and reviewers is proposed.
- Graham Rapid COVID-19 vaccine developmentexternal icon. Science. The CoV spike protein on the virus surface is a target for a COVID-19 vaccine. Safety pitfalls must be avoided to achieve the fastest path to an effective, safe vaccine.
COVID-19 and Violence
- Sutherland et al. Gun violence during COVID-19 pandemic: Paradoxical trends in New York City, Chicago, Los Angeles and Baltimoreexternal icon. American Journal of Emergency Gun violence in New York, Chicago, and Baltimore has increased in 2020 compared to 2018 and 2019.
- Chandan et al. COVID-19: A public health approach to manage domestic violence is neededexternal icon. Lancet Public Possible increase in domestic violence and child abuse during the pandemic.
Other Topics
- Gibbons Ape researchers mobilize to save primates from coronavirusexternal icon. Science. The ape form of the ACE2 receptor is identical to the human form, so it’s likely apes can be infected. Plans to protect apes from SARS-CoV-2 infection are discussed.
- Cousins S. New Zealand eliminates COVID-19external icon. New Zealand recorded its first day of no new cases of COVID-19. This progress has been attributed to its early strict national lockdown.
Willyard, C. Coronavirus blood-clot mystery intensifiesexternal icon. Nature. Potential reasons for increased clotting seen with COVID-19 and therapeutic options.
Disclaimer: The purpose of the CDC COVID-19 Science Update is to share public health articles with public health agencies and departments for informational and educational purposes. Materials listed in this Science Update are selected to provide awareness of relevant public health literature. A material’s inclusion and the material itself provided here in full or in part, does not necessarily represent the views of the U.S. Department of Health and Human Services or the CDC, nor does it necessarily imply endorsement of methods or findings. While much of the COVID-19 literature is open access or otherwise freely available, it is the responsibility of the third-party user to determine whether any intellectual property rights govern the use of materials in this Science Update prior to use or distribution. Findings are based on research available at the time of this publication and may be subject to change.