COVID-19 Science Update released: May 15, 2020 Edition 13

The COVID-19 Science Update summarizes new and emerging scientific data for public health professionals to meet the challenges of this fast-moving pandemic. Weekly, staff from the CDC COVID-19 Response and the CDC Library systematically review literature in the WHO COVID-19 databaseexternal icon, and select publications and preprints for public health priority topics in the CDC Science Agenda for COVID-19 and CDC COVID-19 Response Health Equity Strategy.
Here you can find all previous COVID-19 Science Updates.
PEER-REVIEWED
Case fatality rate in patients with COVID-19 infection and its relationship with length of follow upexternal icon. Rossi et al. Journal of Clinical Virology (April 14, 2020).
Key findings:
- Case fatality rate (CFR) among COVID-19 patients increased with longer follow-up times (Figure).
- This increase likely reflects more complete reporting of deaths attributable to COVID-19 as time passes.
Methods: CFR was estimated for a region in Italy for COVID-19 cases reported during February and March 2020, overall and by length of follow up time (days since diagnosis). Among patients who either had ≥22 days of follow-up or died earlier, time to death was reported. Limitations: CFR may have been overestimated due to severe COVID-19 cases being prioritized for testing.
Implications: COVID-19 CFR could be underestimated if follow-up times are not long enough to capture COVID-19 related deaths.
Figure:
Note: Adapted from Rossi et al. Estimated case fatality rates, by number of days of follow-up, based on number of days since date of diagnosis. This article was published in Journal of Clinical Virology, Vol 128, Rossi et al., Case fatality rate in patients with COVID-19 infection and its relationship with length of follow up, Page 104415, Copyright Elsevier 2020. This article is currently available at the Elsevier COVID-19 resource center: https://www.elsevier.com/connect/coronavirus-information-centerexternal icon.
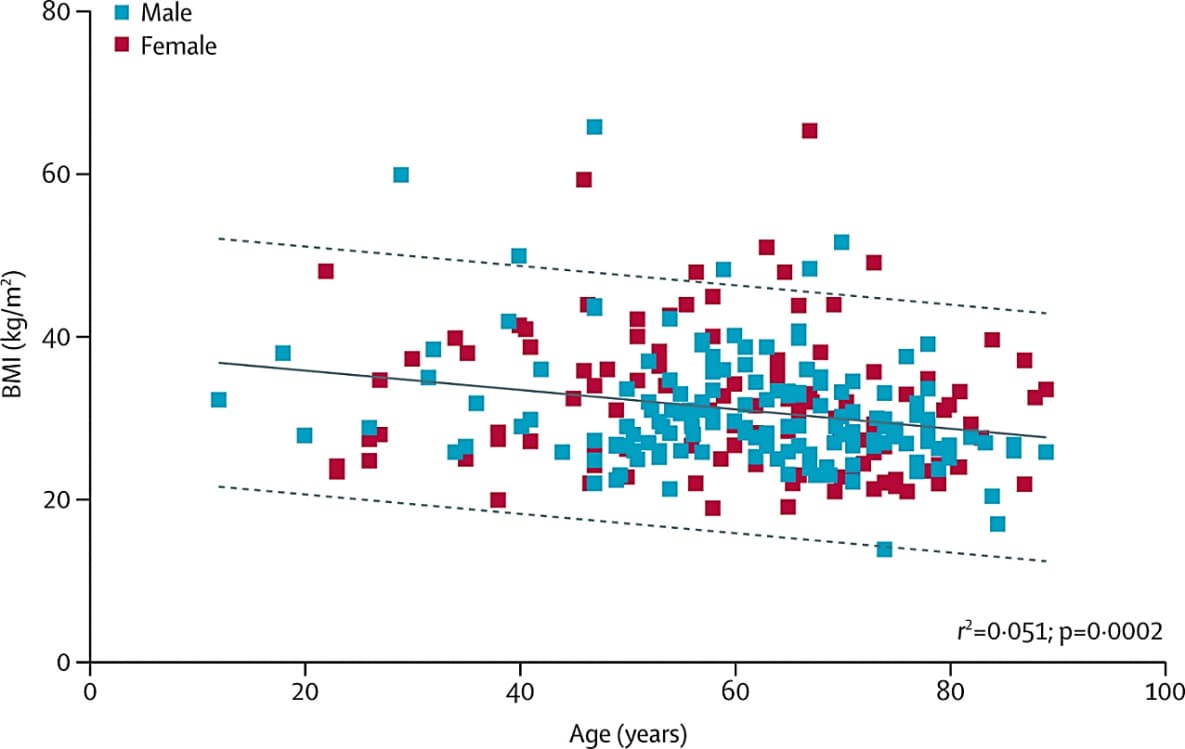
Obesity could shift severe COVID-19 disease to younger agesexternal icon. Kass et al. Lancet (May 4, 2020).
Key findings:
- Among COVID-19 patients, the median body mass index (BMI) was 29.3 kg/m² (overweight):
- 25% of patients had a BMI <26.0 kg/m² (overweight)
- 25% had a BMI >34.7 kg/m² (severely obese).
- Younger individuals admitted to the ICU were more likely to be obese (BMI ≥30 kg/m2) (Figure). No difference was observed by sex (p = 0.9).
Methods: A cross-sectional study to assess the correlation between BMI and age in 265 COVID-19 patients admitted to the ICU in hospitals in six US states. 58% of these patients were male. Least squares univariate and multivariate linear regression analyses were conducted. Limitations: Cross-sectional design.
Implications: Among severe COVID-19 cases admitted to the ICU, those who were younger were more likely to be obese and therefore at greater risk of severe illness and death. Further examination of the relationship between obesity and severity of COVID-19 in younger patients is needed.
Figure:
Note: From Kass et al. Negative correlation between BMI and age in 265 male and female patients with COVID-19 in ICUs in six US states. The solid line is the least squares linear regression model fit. Dashed lines are 95% prediction bands. This article was published in Lancet, Vol 395, Kass et al., Obesity could shift severe COVID-19 disease to younger ages, Pages 1544-1545, Copyright Elsevier 2020. This article is currently available at the Elsevier COVID-19 resource center: https://www.elsevier.com/connect/coronavirus-information-centerexternal icon.
PEER-REVIEWED
Changes in contact patterns shape the dynamics of the COVID-19 outbreak in China. Zhang et al. Science (April 29, 2020).
Key findings:
- Social distancing alone dramatically reduced daily contacts and was sufficient to control the COVID-19 epidemic. This finding held irrespective of assumptions regarding susceptibility to infection by age (Figure).
- Proactive school closures were insufficient to interrupt COVID-19 transmission but could decrease peak incidence by 40%-60%.
- Susceptibility to SARS-CoV-2 infection increased with age; compared with persons aged 15-64 years, individuals over 65 years had higher risk of infection (Odds ratio [OR] 1.47 95% CI, 1.12-1.92) whereas children aged 0-14 years had lower risk (OR 34 95% CI, 0.24-0.49).
Methods: Contact survey data for Wuhan (n = 636) and Shanghai (n = 557) before and during the outbreak, as well as contact tracing information from Hunan Province, were used to construct a model to predict the impact of social distancing, school closure, and age on disease transmission. Limitations: The effect of social distancing was estimated alone, without considering factors such as decontamination efforts and use of face masks.
Implications: Social distancing is an effective measure to control COVID-19. School closures were helpful, but alone were insufficient to control the local epidemics. Understanding the interplay between age, contact patterns, social distancing, and susceptibility to infection may help to inform interventions.
Figure:
Note: Adapted from Zhang et al. Effect of contact patterns (change in number of daily contacts) on the epidemic spread. (A) Estimated R0 during the outbreak (mean and 95% CI), as a function of baseline R0. The figure refers to Wuhan and includes the scenarios accounting for the estimated susceptibility to infection by age and assuming that all individuals are equally susceptible to infection. (B) As (A), but for Shanghai. Licensed under CC-BY 4.0.
PEER-REVIEWED
Hyperinflammatory shock in children during COVID-19 pandemic. Riphagen et al. Lancet (May 7, 2020).
Key findings:
- Among 8 children with pediatric multisystem inflammatory syndrome (PMIS) aged 4-14 years, none tested positive for SARS-CoV-2 while in the hospital, but 2 tested positive for SARS-CoV-2 via RT-PCR following discharge; all eventually were antibody positive, indicating previous infection.
- Cases presented with persistent fever, rash, conjunctivitis, swelling, gastrointestinal symptoms, severe drop in blood pressure requiring treatment, and other clinical signs indicative of systemic inflammation.
- Respiratory system involvement was minimal.
- Cardiovascular complications dominated:
- 7 had abnormalities of the heart vessels that put patients at risk of stroke or heart attack and required mechanical ventilation for cardiovascular stabilization.
- 1 died of a stroke.
- All were treated with immunoglobulin to reduce inflammation, antibiotics, and aspirin to limit risk of cardiovascular abnormalities.
Methods: During a 10-day period in April 2020, 8 pediatric cases presented to a UK hospital with PMIS. Authors describe clinical presentation of these cases. Limitations: Small case series; limited generalizability.
Implications: This is the first published report describing cases of PMIS among children with COVID-19. Patients with potential PMIS should be monitored to ensure early detection and prompt treatment.
Interleukin-1 blockade with high-dose anakinra in patients with COVID-19, acute respiratory distress syndrome, and hyperinflammation: a retrospective cohort study. Cavalli et al. Lancet Rheumatology (May 7, 2020).
Key findings:
- Patients who received a high dose of anakinra (an interleukin-1 receptor blocker) were more likely than those who received standard treatment to:
- Have improved respiratory function (72% vs 50%, respectively).
- Survive (90% vs 56%, respectively; p = 0.009) (Figure 1).
- C-reactive protein levels (a marker of hyperinflammation) decreased over time among patients who received anakinra, but not among those who received standard treatment (Figure 2).
- No difference in adverse events occurred between patients who did and did not receive anakinra treatment.
Methods: During March 2020, authors conducted a retrospective cohort study among 45 hospitalized COVID-19 adult patients in Italy who had moderate to severe acute respiratory distress syndrome and hyperinflammation (defined by serum C-reactive protein ≥100 mg/L, ferritin ≥900 ng/mL, or both), and were being managed with non-invasive ventilation outside the ICU. Outcomes were assessed among 16 patients who received standard treatment (200 mg hydroxychloroquine and 400 mg lopinavir with 100 mg ritonavir twice a day orally), and 29 patients who received standard treatment plus a high dose of anakinra (5 mg/kg twice a day intravenously until clinical improvement or an adverse event was recorded). Improvements in respiratory function, survival, and changes in C-reactive protein levels were compared between treatment groups for up to 21 days. Adverse events were also compared between groups. Limitations: Small convenience sample; long-term outcomes not assessed.
Implications: Anakinra, which blocks the inteleukin-1 receptor, may be effective and safe to treat hyperinflammation among COVID-19 patients. Larger randomized clinical trials are needed to confirm this finding.
Figure 1
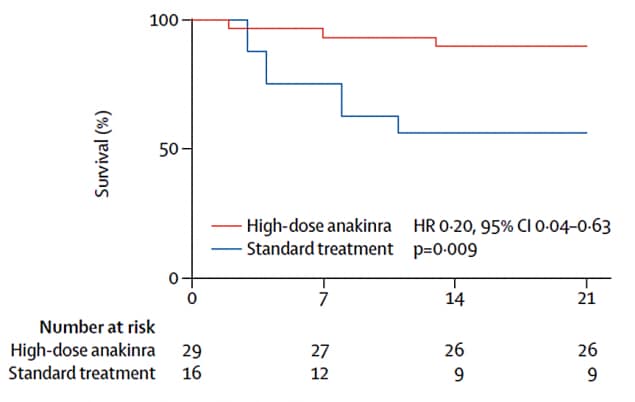
Note: From Cavalli et al. Figure 1. Survival over 21 days among hospitalized COVID-19 patients who received a high-dose of anakinra versus those who received standard treatment; statistical significance assessed using log-rank test (p=0.05). This article was published in Lancet Rheumatology, Vol 2, Cavalli et al., Interleukin-1 blockade with high-dose anakinra in patients with COVID-19, acute respiratory distress syndrome, and hyperinflammation: A retrospective cohort study, Pages e325-e331, Copyright Elsevier 2020. This article is currently available at the Elsevier COVID-19 resource center: https://www.elsevier.com/connect/coronavirus-information-centerexternal icon.
Figure 2A.
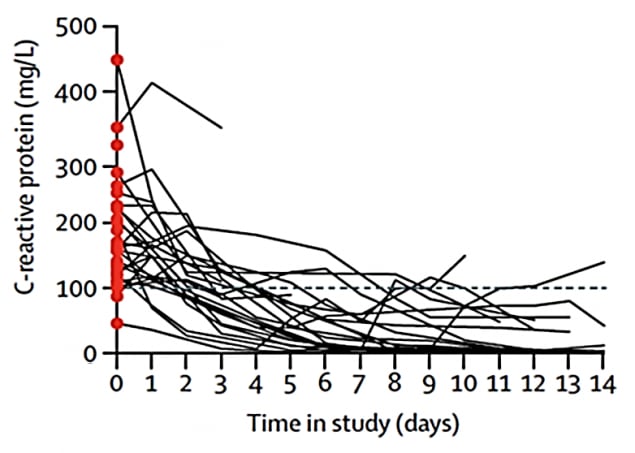
Figure 2B.
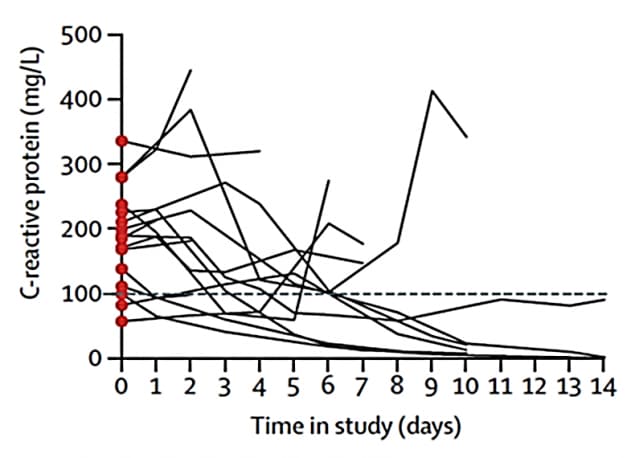
Note: Adapted from Cavalli et al. C-reactive protein levels (a marker of hyperinflammation) over 14 days among hospitalized COVID-19 patients who received high-dose of anakinra (2A) and standard treatment (2B). This article was published in Lancet Rheumatology, Vol 2, Cavalli et al., Interleukin-1 blockade with high-dose anakinra in patients with COVID-19, acute respiratory distress syndrome, and hyperinflammation: A retrospective cohort study, Pages e325-e331, Copyright Elsevier 2020. This article is currently available at the Elsevier COVID-19 resource center: https://www.elsevier.com/connect/coronavirus-information-centerexternal icon.
Association of use of angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers with testing positive for coronavirus disease 2019 (COVID-19)external icon. Mehta et al. JAMA Cardiology (May 7, 2020).
Key findings:
- Use of angiotensin-converting enzyme inhibitors (ACEi) or angiotensin II receptor blockers (ARB), medications typically used to reduce blood pressure, was not associated with testing positive for SARS-CoV-2.
Methods: Retrospective cohort of 18,472 patients tested for SARS-CoV-2; 1,735 (9.4%) tested positive. Investigators assessed associations of clinical information, including use of ACEis/ARBs, with testing positive for SARS-CoV-2 infection and disease severity, accounting for the likelihood of patients taking ACEis/ARBs. Limitations: Most participants were Caucasian; sample size was too small to detect differences in rare clinical outcomes (e.g., ICU admission, mechanical ventilation).
Implications: ACEi or ARB use is not associated with risk of acquiring COVID-19.
Autoimmune hemolytic anemia associated with COVID-19 infectionexternal icon. Lazarian et al. British Journal of Hematology (May 6, 2020).
Key findings:
- The median time from first symptom to onset of autoimmune hemolytic anemia (AIHA, the abnormal breakdown of red blood cells by autoantibody attack) was 9 days (range 4 to 13 days).
- At diagnosis, the median hemoglobin level was 70 g/L (range 38-108 g/L). All patients had positive anti-erythrocyte (red blood cell) antibodies and positive direct antiglobulin test results, which indicate that red blood cells circulating in the bloodstream were covered with antibodies.
- Four patients had lymphoid malignancies (leukemia or lymphoma), one had monoclonal gammopathy (presence of an abnormal protein in the blood) of undetermined significance, and one had prostate cancer.
- Five patients received corticosteroid treatment, while the other two received blood cell infusions. All patients were alive and had at least partly recovered at the end of follow-up.
Methods: A case report of seven patients (four male and three female) from six French and Belgian hospitals who developed AIHA during COVID-19 infection. The median age was 62 years (range 61-89 years). All patients had positive oropharyngeal RT-PCR results for SARS-CoV-2 and typical chest CT images. To treat the infection, three patients received hydroxychloroquine and one patient received lopinavir and ritonavir. Limitations: Small number of cases.
Implications: Viral infections are known to trigger autoimmune conditions that deplete red blood cells. AIHA among patients with COVID-19 warrants further investigation.
Severe acute respiratory syndrome coronavirus 2 detection in the female lower genital tractexternal icon. Cui et al. American Journal of Obstetrics and Gynecology (May 4, 2020).
Key findings:
- Among 35 female COVID-19 patients (median age 64 years, interquartile range 56 to 69 years), SARS-CoV-2 RNA was not detected in samples of vaginal fluid or cervical exfoliated cells, but was detected from one patient’s anal swab.
Methods: A case report of 35 female COVID-19 patients from China, of whom 27 tested positive for SARS-CoV-2 RNA by RT-PCR. Three types of samples (vaginal fluid, cervical exfoliated cells, and anal swabs) were obtained and tested for SARS-CoV-2. Limitations: Small sample size; few women included of childbearing age.
Implications: The digestive tract may be a possible transmission route for SARS-CoV-2, while the lower female genital tract is likely not a transmission route; however larger studies are needed.
Globally, the use of antiviral therapies to improve the clinical outcomes of COVID-19 patients is under investigation. The following two studies investigated the use of antiviral therapy, either alone or in combination with other antivirals.
PEER-REVIEWED
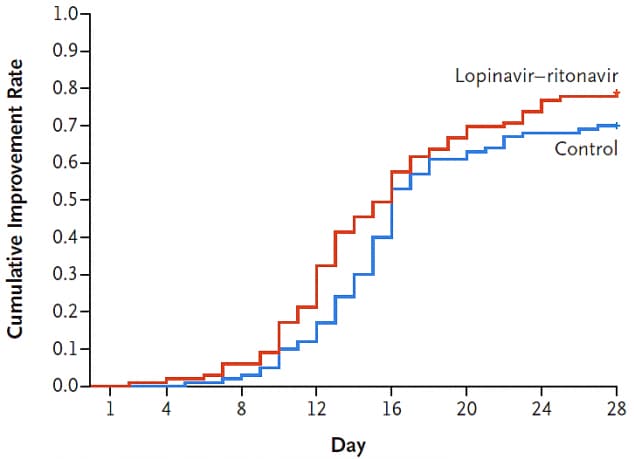
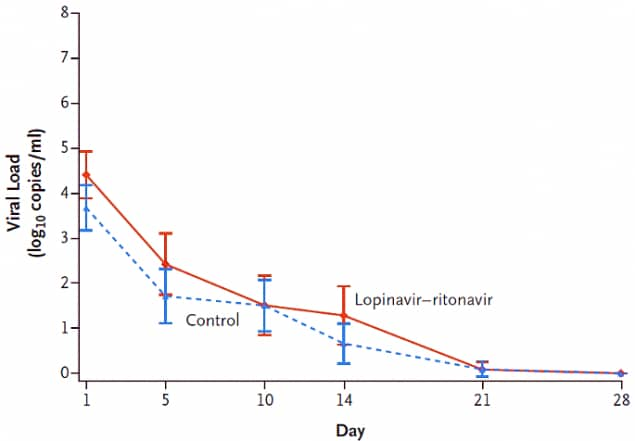
A. A trial of lopinavir-ritonavir in adults hospitalized with severe COVID-19external icon. Cao et al. NEJM (May 7, 2020).
Key findings:
- In an intent-to-treat analysis of patients with severe illness who initiated treatment a median of 13 days after illness onset, those who received lopinavir-ritonavir (vs standard of care alone) experienced no significant differences in clinical improvement (Figure 1) or reductions in viral load over time (Figure 2).
Methods: Open-label, randomized controlled clinical trial of hospitalized adult COVID-19 patients with severe illness: 95 patients received lopinavir-ritonavir and 100 received standard of care. Primary outcomes included time to clinical improvement; secondary outcomes included change in viral load. Limitations: Small sample size that prevented assessment of statistical differences in secondary outcomes; trial unblinded; limited to severely ill patients who started treatment relatively late.
Figure 1.
Figure 2.
Note: Adapted from Cao et al. Neither time to clinical improvement (Figure 1), nor pace of viral load decline (Figure 2), differed between COVID-19 patients treated with lopinavir-ritonavir and standard of care (controls). From NEJM. 382:1787-1799 DOI: 10.1056/NEJMoa2001282. Copyright ©2020 Massachusetts Medical Society. Reprinted with permission from Massachusetts Medical Society.
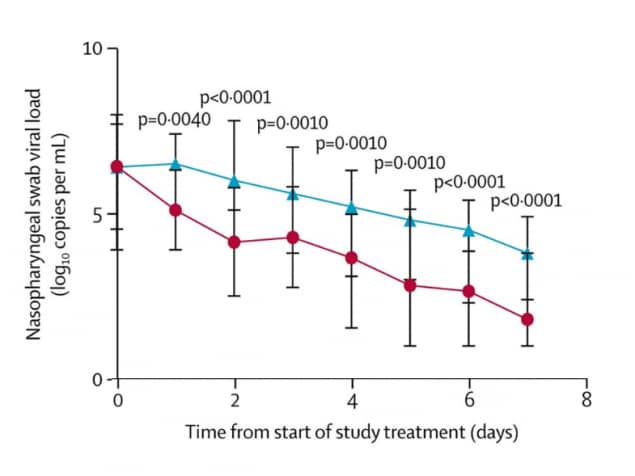
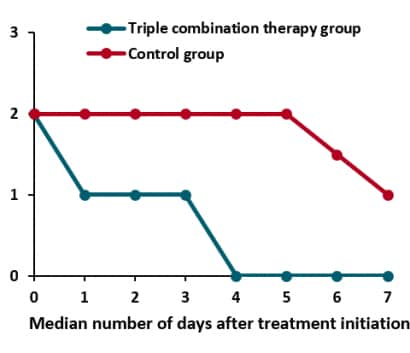
B. Triple combination of interferon beta-1b, lopinavir–ritonavir, and ribavirin in the treatment of patients admitted to hospital with COVID-19: An open-label, randomised, phase 2 trialexternal icon. Hung et al. Lancet (May 8, 2020).
Key findings:
- In an intent-to-treat analysis of patients with mild-to-moderate illness who initiated treatment soon after illness, those patients who received interferon beta-1b, lopinavir-ritonavir, and ribavirin vs standard of care alone achieved an undetectable SARS-CoV-2 RNA viral load sooner (7 vs 12 days, p <0.001) (Figure 1), had a shorter hospital stay (9 vs 15 days, p <0.003), and resolved symptoms more quickly (4 vs 8 days, p <0.001) (Figure 2).
- Side effects of treatment did not differ between the groups and no serious side effects or deaths occurred during the study.
Methods: Multicenter, open-label, phase 2 clinical trial for 127 adult COVID-19 patients with mild-to-moderate illness randomized 2:1 to receive standard of care with triple combination therapy (interferon beta-1b, lopinavir-ritonavir, and ribavirin) (n = 86) or only lopinavir-ritonavir within 48 hours of hospital admission. The primary endpoint was time to achieve a negative RT-PCR result for SARS-CoV-2 RNA in a nasopharyngeal swab sample. Secondary endpoints included time to resolution of symptoms defined as a NEWS2 (see Note, below) of 0 maintained for 24 hours. Limitations: No placebo group; excluded severe cases.
Figure 1.
Figure 2.
Note: Adapted from Hung et al. Figure 1. Reduction in viral load over time for COVID-19 patients treated with triple combination therapy compared to the control group. Figure 2. The National Early Warning Score (NEWS2 on the y-axis) uses clinical information to grade patient illness, and scores can range from 0-20. COVID-19 patients treated with triple combination therapy were symptom free by day 4, and some patients in the control group still had symptoms on day 7. This article was published in Lancet, Vol 395, Hung et al., Triple combination of interferon beta-1b, lopinavir–ritonavir, and ribavirin in the treatment of patients admitted to hospital with COVID-19: An open-label, randomised, phase 2 trial, Pages 1695-1704, Copyright Elsevier 2020. This article is currently available at the Elsevier COVID-19 resource center: https://www.elsevier.com/connect/coronavirus-information-centerexternal icon.
Implications of 2 studies (Cao et al. & Hung et al.): Treatment of severely ill patients with lopinavir-ritonavir late in the course of illness had no statistically significant impact on morbidity or mortality. However, addition of interferon beta-1b and ribavirin to lopinavir-ritonavir to treat mild-to-moderately ill patients soon after symptom onset significantly improved time to clinical improvement and discharge from care. Further studies with larger sample sizes are needed.
- Sethuraman et al. Interpreting diagnostic tests for SARS-CoV-2external icon. JAMA. Describes the two types of diagnostic tests used for SARS-CoV-2 infections—reverse transcriptase–polymerase chain reaction (RT-PCR, detects viral RNA), and enzyme-linked immunosorbent assays (ELISA, detects antibodies and nucleocapsid antigen). See figure below that depicts one group’s estimation of the dynamics of infection and the human immune response based on current knowledge (nucleocapsid antigen detection not depicted).

a Detection only occurs if patients are followed up proactively from the time of exposure.
b More likely to register a negative than a positive result by PCR of a nasopharyngeal swab.
Reproduced with permission from JAMA. doi:10.1001/jama.2020.8259. Copyright©2020 American Medical Association. All rights reserved.
- Cuadrado-Payán, et al. SARS-CoV-2 and influenza virus co-infectionexternal icon. Lancet. Clinical presentation of 4 cases of SARS-CoV-2 and influenza co-infection; one patient did not undergo treatment or suffer complications while the other 3 required mechanical ventilation.
- Nott D. The COVID-19 response for vulnerable people in places affected by conflict and humanitarian crisesexternal icon. Lancet. Highlights the lack of infrastructure in conflict areas, and the urgent need to strengthen the COVID-19 response in such places.
- Riblet et al. A pandemic of body, mind, and spirit: the burden of “social distancing” in rural communities during an era of heightened suicide riskexternal icon. Journal of Rural Health. Calls for heightened attention to suicide risk among rural populations in the context of the COVID-19 pandemic.
- Khullar et al. COVID-19 and the financial health of US hospitalsexternal icon. JAMA. COVID-19 pandemic will cause almost all hospitals to experience financial difficulties, with smaller, independent and rural hospitals being particularly at risk.
- Marston et al. Community participation is crucial in a pandemicexternal icon. Lancet. Describes steps to engage community in the COVID-19 response.
- Parker et al. Ethics of instantaneous contact tracing using mobile phone apps in the control of the COVID-19 pandemicexternal icon. Journal of Medical Ethics. Outlines ethical considerations to be addressed when using mobile phone apps to support contact tracing.
- Mahase E. South Korea relaxes social distancing after the number of new cases drops below 10 a dayexternal icon. BMJ. In May, South Korea eased social distancing measures and allowed businesses to open; in the “new normal”, people need to utilize infectious disease prevention practices.
- Painter K. US ventilator crisis brings patients and doctors face-to-face with life-or-death choicesexternal icon. BMJ. Discusses implications for care when resources (e.g., ventilators) are scarce in crisis situations and life-or-death choices need to be made.
- Persad et al. The ethics of COVID-19 immunity-based licenses (“Immunity Passports”)external icon. JAMA. Examines ethical considerations and implementation challenges of “immunity-based licenses”.
- Kramer et al. CPR in the COVID-19 era — An ethical frameworkexternal icon. NEJM. Proposes an ethical framework and recommendations for inpatient CPR under crisis situations, like the COVID-19 pandemic.
Disclaimer: The purpose of the CDC COVID-19 Science Update is to share public health articles with public health agencies and departments for informational and educational purposes. Materials listed in this Science Update are selected to provide awareness of relevant public health literature. A material’s inclusion and the material itself provided here in full or in part, does not necessarily represent the views of the U.S. Department of Health and Human Services or the CDC, nor does it necessarily imply endorsement of methods or findings. While much of the COVID-19 literature is open access or otherwise freely available, it is the responsibility of the third-party user to determine whether any intellectual property rights govern the use of materials in this Science Update prior to use or distribution. Findings are based on research available at the time of this publication and may be subject to change.
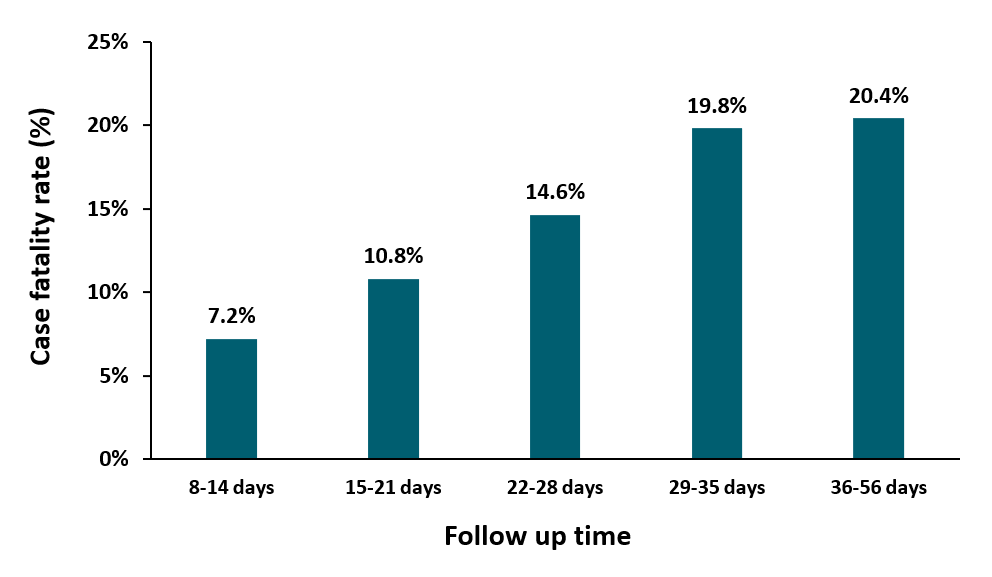
![0515_Figure_3 Effect of contact patterns (change in number of daily contacts) on the epidemic spread. (A) Estimated R0 during the outbreak (mean and 95 percent CI), as a function of baseline R0 (i.e., that derived by using the contact matrix [mean number of contacts that an individual in a given age group has with other individuals, stratified by age groups] estimated for the baseline period). The figure refers to Wuhan and includes the scenario accounting for the estimated susceptibility to infection by age and assuming that all individuals are equally susceptible to infection. (B) As (A), but for Shanghai.](/library/covid19/images/0515_Figure_3-large.png?_=62306)