COVID-19 Science Update released: April 28, 2020 Edition 8

The COVID-19 Science Update summarizes new and emerging scientific data for public health professionals to meet the challenges of this fast-moving pandemic. Weekly, staff from the CDC COVID-19 Response and the CDC Library systematically review literature in the WHO COVID-19 databaseexternal icon, and select publications and preprints for public health priority topics in the CDC Science Agenda for COVID-19 and CDC COVID-19 Response Health Equity Strategy.
Here you can find all previous COVID-19 Science Updates.
PEER-REVIEWED
Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City areaexternal icon. Richardson et al. JAMA (April 22, 2020; Correction external iconon April 24, 2020).
Key findings:
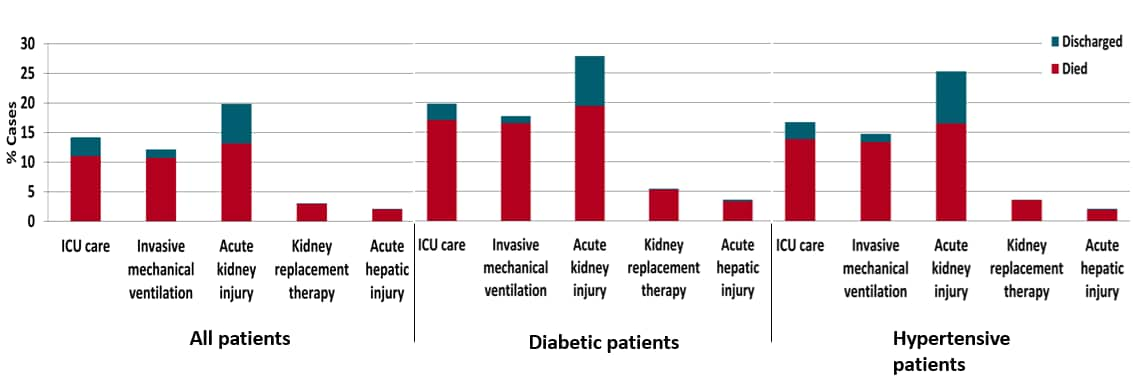
- Mortality among COVID-19 patients who completed their hospitalization or died was 21%.
- 88% of patients who received invasive mechanical ventilation died, including 97% of those aged >65.
- Most ICU patients and patients with invasive mechanical ventilation, acute kidney injury, kidney replacement therapy, and acute hepatic injury died (Figure).
- Mortality rates were higher for men than women regardless of age.
Methods: Sequential case series of all COVID-19 patients admitted to 12 hospitals in New York City, Long Island, and Westchester County, New York, between March 1 and April 4, 2020 (N = 5,700). Outcomes were assessed for 2,634 patients who were discharged or had died at the study end point. Limitations: Only included patients in one health system; no adjustment for potential confounders; time for intubation to death not reported so impossible to understand risk of imminent death at time of hospital presentation (late presentation) vs in-hospital risk.
Implications: In one New York City health system, 1 in 5 hospitalized COVID-19 patients died, including most ICU patients and 7 of 8 that required mechanical ventilation.
Figure:
Note: Adapted from Richardson et al. Clinical outcomes among patients discharged alive or dead at study end point, by comorbidity. Bar total is the percentage of patients in category with clinical outcome. Bars are split by percentage of patients that died or were discharged. Reproduced with permission from JAMA. doi:10.1001/jama.2020.6775. Copyright©2020 American Medical Association. All rights reserved.
Symptom screening at illness onset of health care personnel with SARS-CoV-2 infection in King County, Washingtonexternal icon. Chow et al. JAMA (Apr 17, 2020).
Key findings:
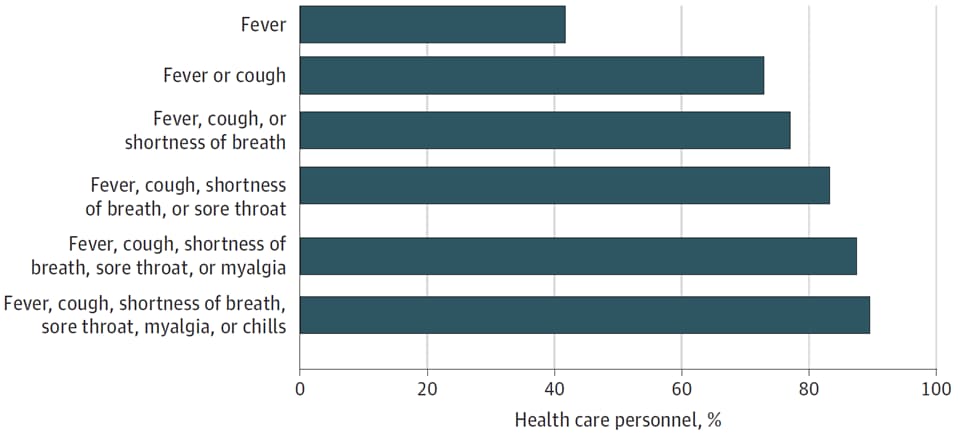
- 65% of healthcare personnel (HCP) with COVID-19 reported working 1-10 days while having symptoms.
- Among symptomatic HCP diagnosed with COVID-19, >20% would have been clinically misclassified had screening relied on only three variables: fever, cough, or shortness of breath.
Methods: 48 HCP with laboratory-confirmed SARS-CoV-2 infection in King County, WA were interviewed about symptoms of COVID-19 and work attendance. Limitations: Symptoms reported after illness, which may impact recall.
Implications: Expanding symptom-based screening criteria will detect more HCP with early disease and may lead to decreased transmission in healthcare facilities.
Figure:
Note: Adapted from Chow et al. Symptoms among healthcare personnel with COVID-19 (N = 48). Reproduced with permission from JAMA. doi:10.1001/jama.2020.6637. Copyright©2020 American Medical Association. All rights reserved.
PEER-REVIEWED
The characteristics of household transmission of COVID-19external icon. Li et al. Clinical Infectious Diseases (April 17, 2020).
Key findings:
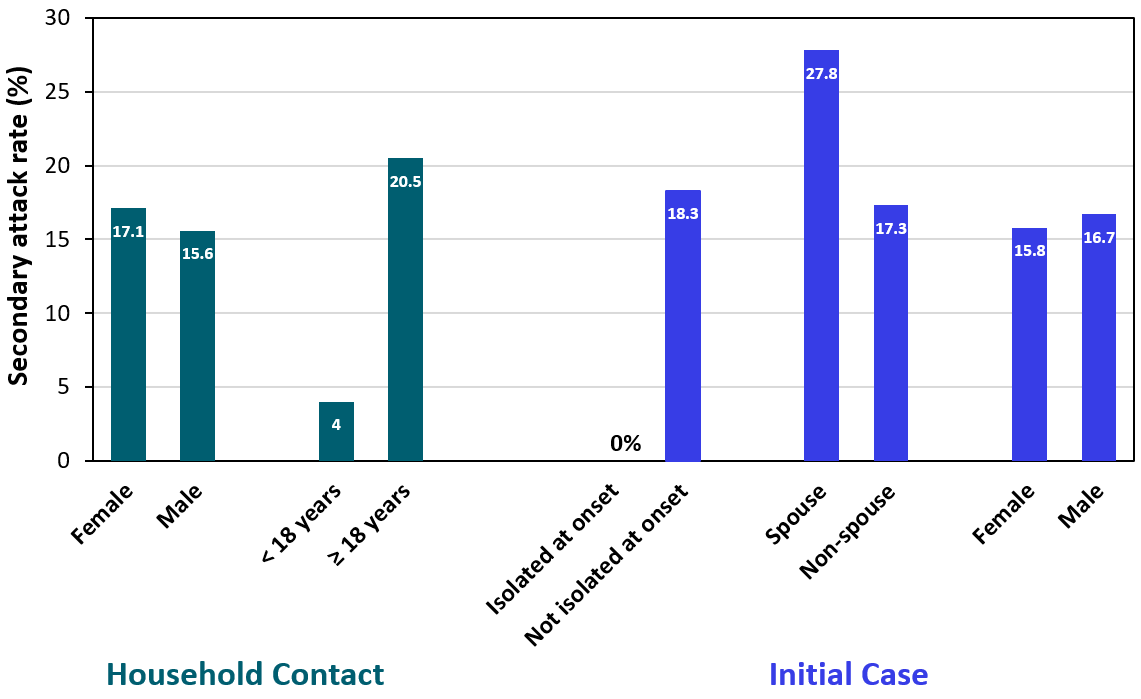
- Household transmission was prevented when the initial case was isolated at home between symptom onset and hospitalization (0%) compared to those who were not (18.3%) (Figure).
- Transmission was higher for the spouses of initial cases (27.8%) compared with other adults in the household (17.3%).
- Transmission to children (4.0%) was lower than to adults (17.1%).
Methods: Retrospective household cohort study of 105 hospitalized COVID-19 patients and 392 household contacts using data from two locations in China. Following confirmation of an initial case, all household contacts were quarantined for 14 days at a location outside the home where they were monitored daily and tested serially for SARS-CoV-2. Limitations: convenience sample; quarantining procedures not clearly defined.
Implications: Early isolation of index cases at home, followed by relocation of household contacts to alternative temporary housing, helped prevent SARS-CoV-2 transmission.
Figure:
Note: Adapted from Li et al. Secondary attack rate of household contacts by characteristics of household contact and initial case. Isolated at onset = index case isolated at home at the time of symptom onset. Adapted and reproduced by permission of Oxford University Press on behalf of the Infectious Diseases Society of America. Please visit: https://academic.oup.com/cid/advance-article/doi/10.1093/cid/ciaa450/5821281external icon. OUP and the IDSA are not responsible or in any way liable for the accuracy of the adaptation. Licensee is solely responsible for the adaptation in this publication/reprint.
No evidence of SARS-CoV-2 in semen of males recovering from COVID-19.external icon Pan et al. Fertility and Sterility (April 17, 2020).
Key findings:
- SARS-CoV-2 RNA was not detected in semen from 34 men who recovered from COVID-19, of whom 6 reported scrotal discomfort at the time of diagnosis.
- Low expression of SARS-CoV-2 receptors (ACE2 and TMPRSS2) in testicular cells.
Methods: Observational, cross-sectional study; 34 adult men in Wuhan with confirmed COVID-19 provided one ejaculated semen sample (median time since diagnosis: 31 days; IQR: 29-36 days). RNA gene expression levels of ACE2 and TMPRSS2 examined from prior dataset of testicular cells from young, healthy adults. Limitations: Selection bias, participants had mostly mild COVID-19 symptoms; most samples collected >14 days after diagnosis (i.e., no data provided on viral shedding during illness).
Implications: Men who have recovered are unlikely to transmit SARS-CoV-2 via semen.
PEER-REVIEWED
Endothelial cell infection and endotheliitis in COVID-19external icon. Varga et al. Lancet (April 17, 2020).
Key findings:
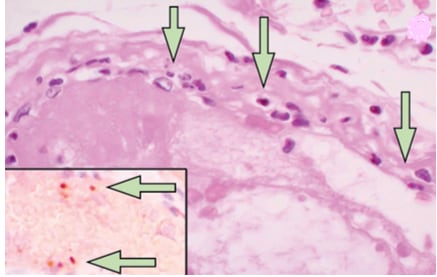
- Among COVID-19 patients who died, viral particles, an accumulation of inflammatory cells, and cell death were found in the lining of blood vessels (endothelium) in kidney, heart, liver, and small intestine (Figure).
Methods: Investigators in Zurich, Switzerland and Boston, MA performed post-mortem examinations with standard and electron microscopy of tissue from the organs of 3 patients with COVID-19. Limitations: Small case series.
Implications: Endothelial cell injury in patients with COVID-19 could explain dysfunction in several organs and inform potential therapies to protect endothelium while tackling viral replication. Clinical trials of such therapies are needed.
Figure:
Note: Adapted from Vargas et al. Small intestine. Arrows in main image point to mononuclear (inflammatory) cells within the endothelium of many blood vessels, which normally should not be there in otherwise healthy persons. Inset shows proteins associated with endothelial cell death (caspase 3). This article was published in Lancet, Vol 395, Varga et al., Endothelial cell infection and endotheliitis in COVID-19, Pages 1417-1418, Copyright Elsevier 2020. This article is currently available at the Elsevier COVID-19 resource center: https://www.elsevier.com/connect/coronavirus-information-centerexternal icon.
PREPRINTS (NOT PEER-REVIEWED)
Clinical benefit of remdesivir in rhesus macaques infected with SARS-CoV-2external icon. Williamson et al. bioRxiv (Apr 22, 2020). Publishedexternal icon in Nature (June 9,2020).
Key findings:
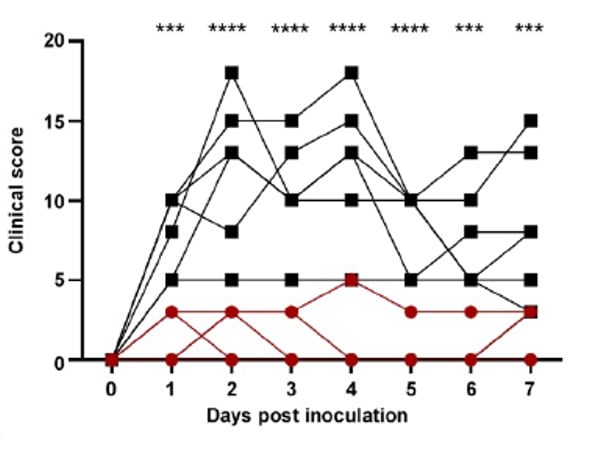
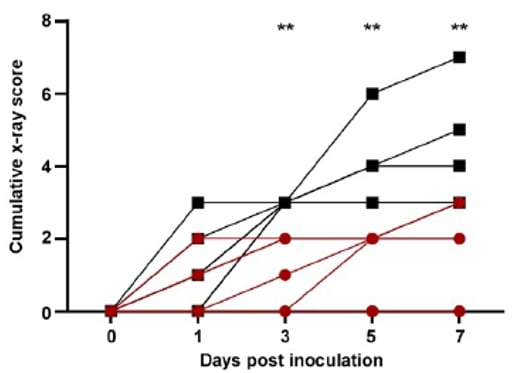
- Rhesus macaque monkeys treated with remdesivir experienced less illness (Figure 1) and had less evidence of disease on lung X-ray (Figure 2) than untreated controls.
- At necropsy, treated animals (compared with controls) also had:
- Lower viral RNA concentrations in the lungs but equal amounts in the nose, throat, and rectum.
- Minimal to no lung disease, whereas 5 of 6 controls had pneumonia.
Methods: 12 rhesus macaques were inoculated with SARS-CoV-2. Then 12 hours later, 6 were given intravenous remdesivir and the others 6 an equal volume of vehicle solution (controls). Additional doses were given 12 hours later and then every 24 hours until day 7. Limitations: Only 12 rhesus macaque monkeys studied; some coauthors are employed by the manufacturer.
Implications: Remdesivir treatment reduced disease severity in non-human primates with SARS-CoV-2. Data from human clinical trials are needed.
Figure 1:
Figure 2:
Note: Adapted from Williamson et al. Figure 1 shows daily clinical scores and Figure 2 shows x-ray scores for animals treated with remdesivir or controls (lower score=healthier for both figures). ** p <0.01; *** p <0.001; **** p <0.0001. US Government work not subject to copyright.
Angiotensin converting enzyme inhibitors (ACEI) and angiotensin II receptor blockers (ARB) are common hypertension medications, especially among people with diabetes and cardiovascular disease.
PEER-REVIEWED
A. Association of inpatient use of angiotensin converting enzyme inhibitors [ACEI] and angiotensin II receptor blockers [ARB] with mortality among patients with hypertension hospitalized with COVID-19external icon. Zhang et al. Circulation Research (Apr 17, 2020).
Key findings:
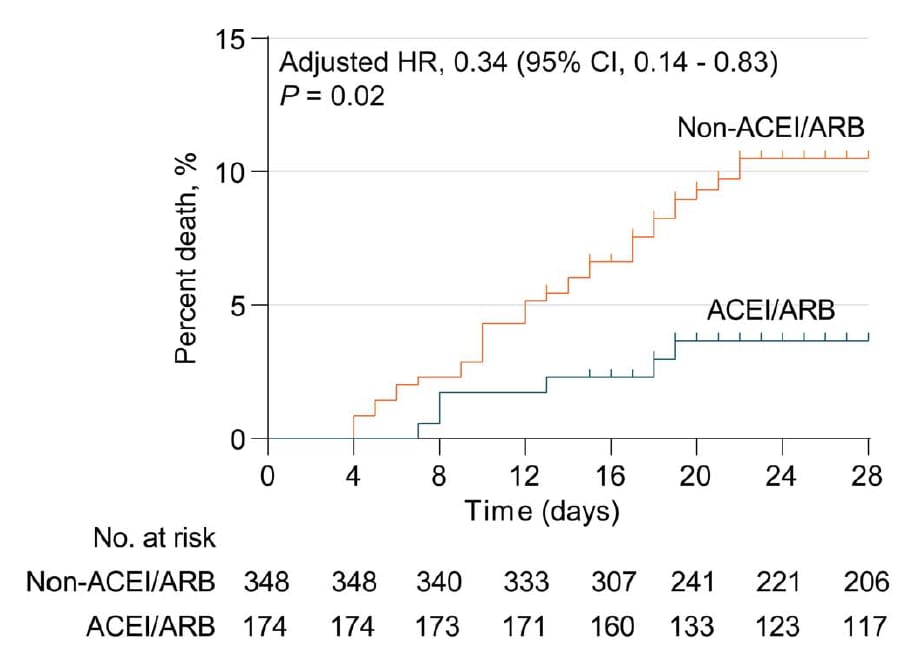
- Among COVID-19 patients with hypertension, in-hospital use of ACEI/ARB antihypertensive medications was associated with lower 28-day all-cause mortality and lower risk of septic shock vs those who did not (Figure).
Methods: Retrospective, multi-center study of 1,128 adults hospitalized with COVID-19 who had a documented medical history of systolic blood pressure ≥140 mm Hg or diastolic blood pressure ≥90 mm Hg. 188 patients (17%) received and 940 (83%) did not receive ACEI/ARB medications during hospitalization (including 383 [34%] with no antihypertensive medications). Cox proportional hazards models with propensity scoring measured the association of ACEI/ARB use with death. Models included only patients taking antihypertensive medication and were adjusted for demographics, symptoms, other in-hospital medication use, and comorbidities. Limitations: Did not have power to detect different effect of ACEI and ARB; unable to account for impact of pre-hospital medications.
Figure:
Note: From Zhang et al., Figure shows Kaplan-Meier Curves for cumulative probability of mortality during 28-day follow up of ACEI/ARB (n = 188) and non-ACEI/ARB (n = 940) groups in propensity score-matched Cox model (Figure 1). Available via Wolters Kluwer Public Health Emergency Collection through PubMed Central.
B. Association of renin-angiotensin system inhibitors with severity or risk of death in patients with hypertension hospitalized for coronavirus disease 2019 (COVID-19) infection in Wuhan, Chinaexternal icon. Li et al. JAMA Cardiology (April 23, 2020; Correctionexternal icon on June 17,2020).
Key findings:
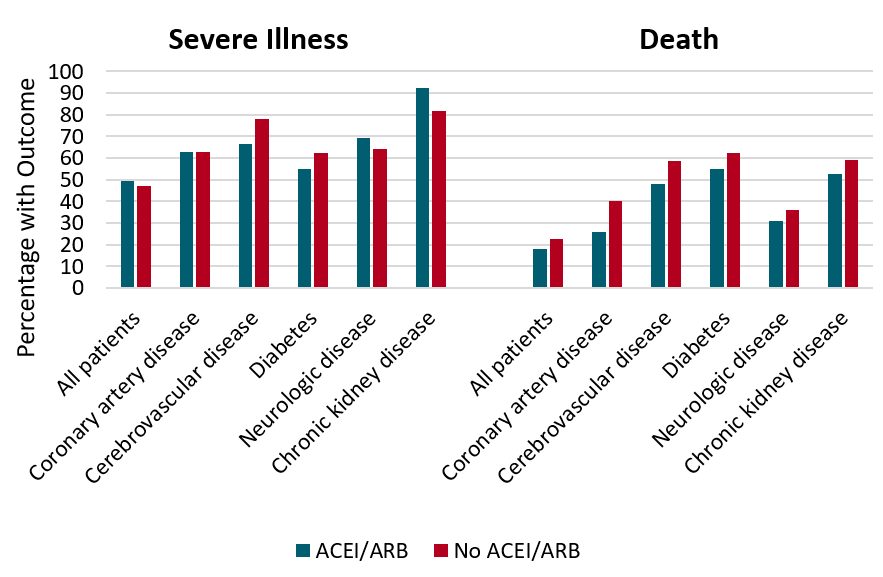
- Among COVID-19 patients with hypertension, use of ACEI/ARB antihypertensive medications at time of admission and continued through hospitalization (vs. no ACEI/ARBs) was not associated with disease severity or death (Figure).
- Findings were similar when assessing ACEI and ARB medication use separately.
Methods: Retrospective, single-center study of 362 adults with COVID-19 who had a history of systolic blood pressure ≥140 mm Hg, diastolic blood pressure ≥90 mm Hg, or who had a history of antihypertensive treatment. 115 patients (32%) received ACEI/ARB during hospitalization, and 247 patients (68%) did not including 65 (18%) who received no antihypertensive medication. Association of receiving ACEI/ARB with severity of COVID-19 and death assessed with descriptive statistics. Limitations: Did not adjust for higher prevalence of coronary artery disease (undefined) among patients receiving vs. not receiving ACEI/ARB medications (24% vs. 14%, respectively) which may have confounded the association of ACEI/ARB use with COVID-19 severity and death.
Figure:
Note: Adapted from Li et al. Percentages of severe illness and death among hospitalized COVID-19 patients with chronic diseases by in-hospital ACEI/ARB use (n = 115) or non-use (n = 247). No differences were statistically significant. Reproduced with permission from JAMA. doi:10.1001/jama.2020.1624. Copyright©2020 American Medical Association. All rights reserved.
Implications of both studies (Zhang et al. & Li et al.): Among COVID-19 patients with hypertension, use of ACEI/ARBs vs. other or no antihypertensives does not appear to increase risk of mortality. Evidence of whether these drugs decrease risk of mortality is unclear. Findings support current US recommendations to not stop, start, or adjust the dose of ACEIs/ARBs in persons with COVID-19.
PEER-REVIEWED
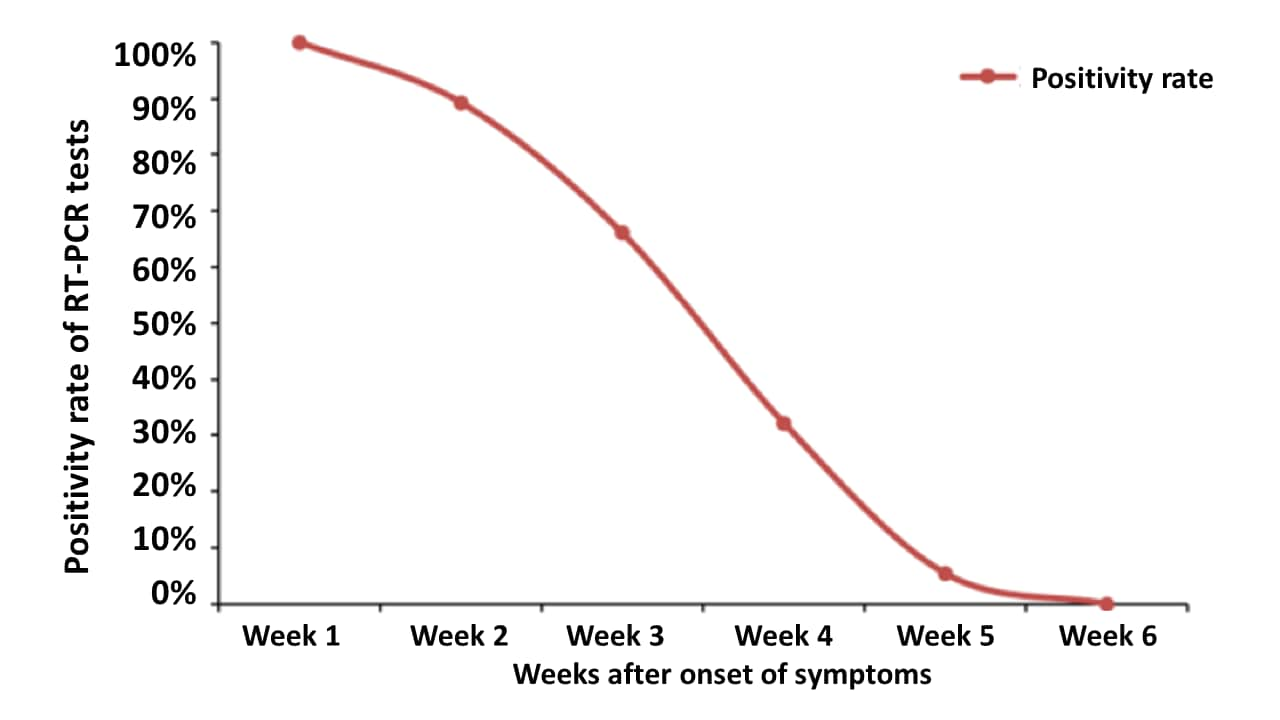
Profile of RT-PCR for SARS-CoV-2: A preliminary study from 56 COVID-19 patientsexternal icon. Xiao et al. Clinical Infectious Diseases (April 19, 2020).
Key findings:
- Among patients with mild to moderate COVID-19 disease, SARS-CoV-2 RNA shedding from the upper respiratory tract persisted for up to six weeks after onset of symptoms in some cases.
- Median duration of shedding was 24 days (interquartile range: 18-31).
- Patients with prolonged viral shedding (i.e., >24 days vs ≤24 days) were significantly:
- Older (median age 54).
- More likely to have diabetes and hypertension.
- 16 patients had a positive test following a negative test, including 4 patients with recurrence after 2 consecutive negative tests.
Methods: RT-PCR testing of serial throat and deep nasal samples from 56 hospitalized patients with confirmed mild to moderate COVID-19 in three hospitals in Wuhan, China. Limitations: Patients provided an average of five tests for this analysis, but the number and timing of specimens varied among patients; Ct values not reported.
Implications: Recovered patients can shed SARS-CoV-2 viral RNA for weeks. Based on these data and epidemiological examination, it remains unknown how rapidly the concentrations of detectable RNA decline during shedding, when recovery of culturable virus becomes unlikely, and the extent to which persons with prolonged shedding pose an infectious risk
Note: Adapted from Xiao et al. Profile of SARS-CoV-2 in 299 specimens from 56 COVID-19 patients. Licensed under CC-BY-NC-ND 4.0.
- Edelson et al. Interim guidance for life support for COVID-19external icon. Circulation. American Health Association recommends all rescuers should don PPE, limit personnel in the room, and consider substituting mechanical for manual chest compression.
- Abbasi J. The promise and peril of antibody testing for COVID-19external icon. JAMA. Discusses serologic testing, including implications for immunity, treatment with convalescent plasma, return to work, and regulatory issues.
- Walensky et al. From mitigation to containment of the COVID-19 pandemic: Putting the SARS-CoV-2 genie back in the bottleexternal icon. JAMA. Discusses timing and logistics of resuming normal activities, the role of testing, and protection of vulnerable populations.
- Montoya-Barthelemy et al. COVID-19 and the correctional environment: the American prison as a focal point for public healthexternal icon. American Journal of Preventive Medicine. Discusses risk to prisoners and staff within correctional institutions, its impact on public health, and mitigation strategies.
- Li et al. SARS-CoV-2 and viral sepsis: Observations and hypothesesexternal icon. Lancet. Discusses characteristics and mechanisms of viral sepsis.
- Reardon S. Antibiotic treatment for COVID-19 complications could fuel resistant bacteriaexternal icon. Science. Discusses the possible impact on development of antibiotic resistance.
- Cornwall W. Crushing coronavirus means ‘breaking the habits of a lifetime.’ Behavior scientists have some tipsexternal icon. Science. Notes how government and industry leaders are turning to behavioral scientists for advice on how to persuade people to abide by public health interventions.
- Glynn J. Protecting workers aged 60–69 years from COVID-19external icon. Lancet Infectious Diseases. Highlights the importance of protection of HCWs aged 60–69 years using data from China and the UK.
- Munoz et al.Late-onset neonatal sepsis in a patient with COVID-19external icon. Case report of a 3-week old male requiring mechanical ventilation.
Disclaimer: The purpose of the CDC COVID-19 Science Update is to share public health articles with public health agencies and departments for informational and educational purposes. Materials listed in this Science Update are selected to provide awareness of relevant public health literature. A material’s inclusion and the material itself provided here in full or in part, does not necessarily represent the views of the U.S. Department of Health and Human Services or the CDC, nor does it necessarily imply endorsement of methods or findings. While much of the COVID-19 literature is open access or otherwise freely available, it is the responsibility of the third-party user to determine whether any intellectual property rights govern the use of materials in this Science Update prior to use or distribution. Findings are based on research available at the time of this publication and may be subject to change.