COVID-19 Science Update released: February 12, 2021 Edition 76

The COVID-19 Science Update summarizes new and emerging scientific data for public health professionals to meet the challenges of this fast-moving pandemic. Weekly, staff from the CDC COVID-19 Response and the CDC Library systematically review literature in the WHO COVID-19 databaseexternal icon, and select publications and preprints for public health priority topics in the CDC Science Agenda for COVID-19 and CDC COVID-19 Response Health Equity Strategy.
Section headings in the COVID-19 Science Update have been changed to align
with the CDC Science Agenda for COVID-19.
Here you can find all previous COVID-19 Science Updates.
PEER-REVIEWED
Respiratory and psychophysical sequelae among patients with COVID-19 four months after hospital discharge.external icon Bellan et al. JAMA Network Open (January 27, 2021).
Key findings:
- 113 patients (51.6%) had lung function impairment (diffusing lung capacity for carbon monoxide [Dlco] <80% of expected); 34 of whom (15.5% of total) had severe impairment (Dlco <60% of expected).
- Lung function impairment was more likely in women and patients with chronic kidney disease.
- 41 (17.2%) patients reported moderate (11.3%) or severe (5.9%) posttraumatic stress (PTS) symptoms.
- Women were less likely than men (OR 0.34; 95% CI 0.14-0.84) to report moderate to severe PTS symptoms.
Methods: Prospective cohort study of 238 hospitalized adults with severe COVID-19 in Northern Italy from March 1 to June 29, 2020; 219 patients completed pulmonary function tests (PFTs) and Dlco measurement. PTS symptoms were assessed using the Impact of Event Scale–Revised total score. Limitations: Potential selection bias given high refusal rate. Pre–COVID-19 PFT measurements were not available.
Implications: The substantial proportions of patients with long-term physical or psychological sequelae of COVID-19 support the need for designated follow-up clinics for COVID-19 survivors.
Circulating SARS-CoV-2 spike N439K variants maintain fitness while evading antibody-mediated immunity.external icon Thomson et al. Cell (January 28, 2021).
Key findings:
- Receptor binding motif (RBM, a structure within the receptor binding domain) mutation N439K emerged independently in multiple variants.
- The mutation increases spike affinity for ACE2 receptors while viral fitness and virulence are unchanged from wild-type SARS-CoV-2.
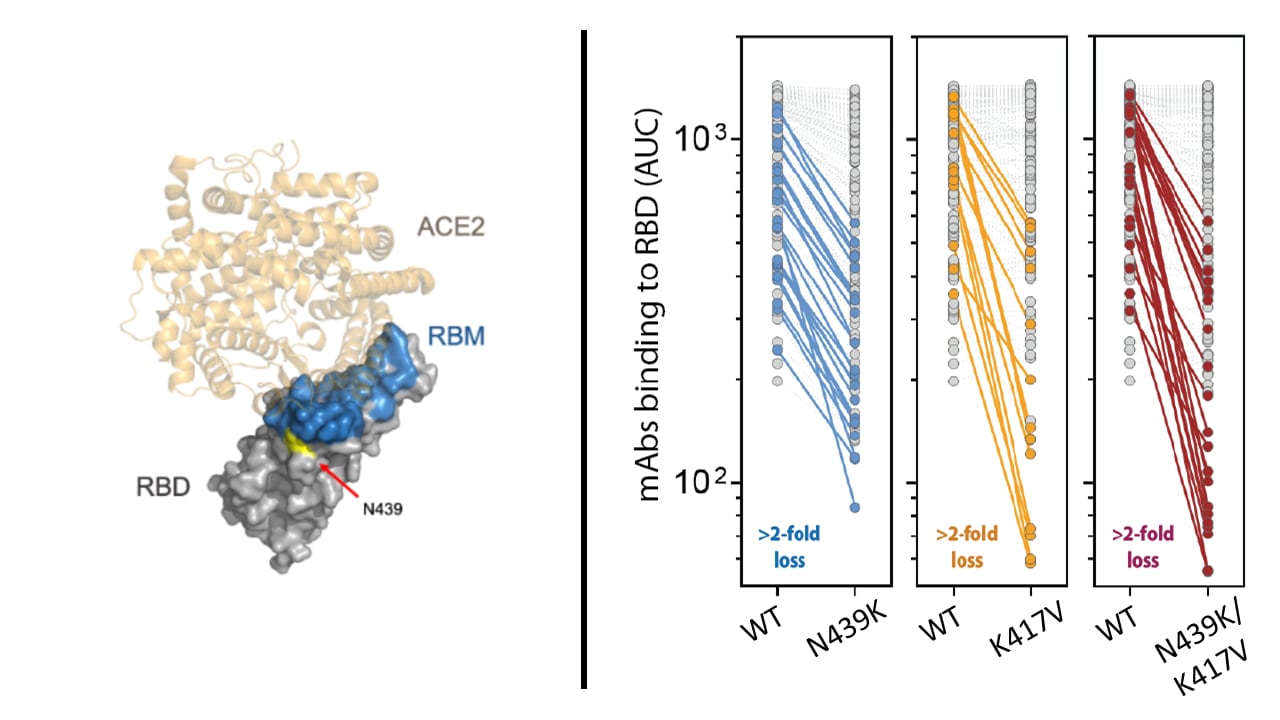
- N439K mutations confer resistance to therapeutic monoclonal antibodies (mAbs) and some polyclonal human sera (Figure).
Methods: N439K variant SARS-CoV-2 structural dynamics, phylogeny, fitness, and virulence were assessed with in silico and in vitro experiments and an observational study. 144 human mAbs and 442 human sera were used in binding assays testing N439K pseudoviruses. Limitations: Findings are not generalizable to other RBM mutants.
Implications: Mutations that evade immunity but maintain viral fitness and virulence, such as the N489K mutation, are emerging. Ongoing molecular surveillance is warranted to identify variants of concern for improved development and usage of vaccines and therapeutics.
Figure.
Note: Adapted from Thomson et al. (Left) Structure of the receptor binding domain (RBD) with receptor binding motif (RBM) and N439 residue shown in complex with ACE2 receptor. (Right) Comparisons of in vitro mAbs binding between wild-type and variant N439K, K417V, and N439K/K417V pseudoviruses. Highlighted points indicate a greater than 2-fold reduction in binding. Licensed under CC BY.
PEER-REVIEWED
COVID-19 symptoms and SARS-CoV-2 antibody positivity in a large survey of first responders and healthcare personnel, May–July 2020.external icon Akinbami et al. Clinical Infectious Diseases. (January 30, 2021).
Key findings:
- Although only 8% of participants reported loss of taste/smell, adjusted seroprevalence was highest among those who reported this symptom (55.6%, 95% CI 53.5%-57.7%).
- Adjusted seroprevalence among asymptomatic participants (14.5%, 95% CI 13.9%-15.1%) was similar to that of participants reporting diarrhea (14.4%, 95% CI 13.6%-15.1%) and higher than that of participants reporting sore throat (12.0%, 95% CI 11.5%-12.5%).
- 22.9% of participants who reported all 9 symptoms were seronegative.
Methods: Serologic survey among 40,938 first responders and healthcare personnel in New York City and the Detroit metropolitan area conducted from May 17–July 2, 2020. Data were collected on 9 COVID-19 symptoms (loss of taste/smell, fever, chills, shortness of breath, muscle aches, cough, headache, diarrhea, and sore throat). Adjusted seropositivity rates were estimated using logistic regression. Limitations: Recall bias; misclassification bias given inability to differentiate between false negative results, loss of antibodies, and failure to develop antibodies.
Implications: SARS-CoV-2 testing more accurately identifies SARS-CoV-2 infection than syndromic surveillance.
PEER-REVIEWED
Transmission of COVID-19 in 282 clusters in Catalonia, Spain: a cohort study.external icon Marks et al. The Lancet Infectious Diseases. (February 2, 2021).
Key findings:
- The secondary attack rate increased from 12% when the index case had viral load <1 x 106 copies/mL to 24% when the index case had viral load ≥1 x 1010 copies/mL.
- Adjusted risk of transmission was positively associated with viral load of the index case (aOR per log10 increase in viral load 1.29, 95% CI: 1.10-1.50).
- Household exposure (aOR 3.00, 95% CI 1.59-5.65) was associated with increased risk of transmission.
- Reported mask usage of contact and index case respiratory symptoms (e.g. cough), age, and sex were not associated with transmission.
Methods: Cohort study of 282 index cases and 753 contacts who were assessed for symptoms of COVID-19 and other epidemiological information at baseline, and underwent a SARS-CoV-2 RT-PCR test and viral load titration from nasopharyngeal swabs on enrollment, at day 14, and when participant reported onset of COVID-19 symptoms. Limitations: Asymptomatic index cases were excluded.
Implications: Viral load, rather than symptoms, is an important driver of SARS-CoV-2 transmission. Until viral load is measured, all cases should be considered as potential transmitters despite absence of respiratory symptoms on presentation.
PREPRINTS (NOT PEER-REVIEWED)
Genomic epidemiology identifies emergence and rapid transmission of SARS-CoV-2 B.1.1.7 in the United States.external iconWashington et al. medRxiv (February 7, 2021). Published in Cell as Emergence and rapid transmission of SARS-CoV-2 B.1.1.7 in the United Statesexternal icon (May 13, 2021).
Key findings:
- The SARS-CoV-2 variant B.1.1.7 was introduced into the US multiple times in November 2020.
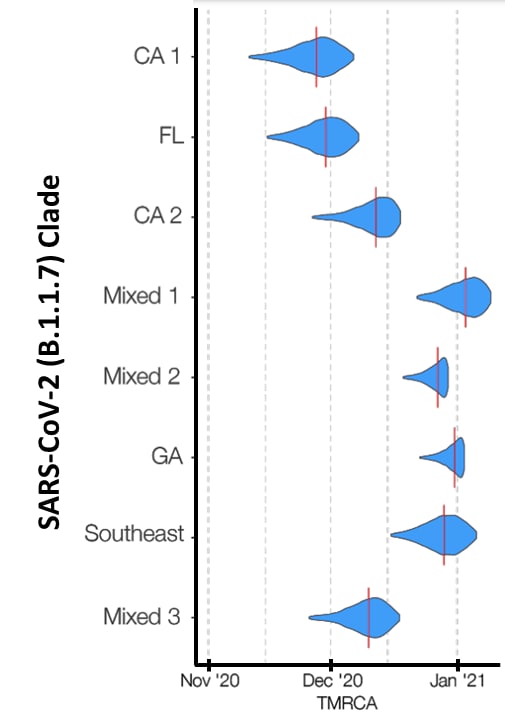
- The earliest occurred in California (Figure).
- Interstate transmission likely began in December 2020.
- B.1.1.7 cases doubled in the US every 9.8 days and spread to 30 states by January 2021.
Methods: Using both RT-PCR test (n = 500,000) and genomic sequencing results (n = 212) from samples collected between November 2020–January 2021, the introduction and transmission of SARS-CoV-2 variant B.1.1.7 were characterized. Transmissibility was assessed using spike gene target failure as a proxy for confirmed B.1.1.7 infections. Phylogenetic analysis was conducted to track the variant spread by clade. Limitations: Testing data covers less than half of US states and territories.
Implications: The full extent of B.1.1.7 circulation may be underestimated, but rapid and comprehensive interventions, including vaccination, physical distancing, and mask wearing, are essential to contain its spread. These data underscore the need for genomic surveillance to monitor dynamics of B.1.1.7 and emerging variants.
Figure.
Note: Adapted from Washington et al. Time to most recent common ancestor (TMRCA) by clade from phylogenetic analysis. 8 clades are shown on the vertical axis. The vertical red lines on the blue distributions indicate the median time to most recent common ancestor, a likely date of introduction. Licensed under CC BY-NC 4.0.
The effect of SARS-CoV-2 variant B.1.1.7 on symptomatology, re-infection and transmissibility.external iconGraham et al. medRxiv (January 29, 2021). Published in The Lancet Public Health as Changes in symptomatology, reinfection, and transmissibility associated with the SARS-CoV-2 variant B.1.1.7: An ecological studyexternal icon (May 2021).
Key findings:
- SARS-CoV-2 variant B.1.1.7 is more transmissible than other co-circulating SARS-CoV-2 viruses but not associated with increased symptoms or disease severity.
- Despite a rise in B.1.1.7 infections, reinfection rates remain low (0.7%, 95% CI 0.6-0.8).
Methods: Symptom, genomic, and test result data prospectively collected from September to December 2020 in the UK were aggregated and compared by region. Self-reported symptoms and tests were collected via a mobile application. Probable B.1.1.7 infections were inferred from spike gene target failures and correlated to genomic sequencing. Transmissibility was estimated with the time-varying reproductive number (Rt). Limitations: Ecological comparisons between data sources that relies on self-reported data.
Implications: SARS-CoV-2 B.1.1.7 is highly transmissible but does not have a clear association with severe outcomes. In contrast, although this study did not examine mortality, Davies et alexternal icon. found a 30% increase in 28-day mortality with B.1.1.7.
PREPRINTS (NOT PEER-REVIEWED)
Single dose administration, and the influence of the timing of the booster dose on immunogenicity and efficacy of ChAdOx1 nCoV-19 (AZD1222) vaccine.external icon Voysey et al. Lancet (February 1, 2021). Published in The Lancet as Single-dose administration and the influence of the timing of the booster dose on immunogenicity and efficacy of ChAdOx1 nCoV-19 (AZD1222) vaccine: a pooled analysis of four randomised trialsexternal icon (February 19, 2021).
Key findings:
- A single standard vaccine dose had 76% (95% CI 59%-86%) efficacy from 22 to 90 days after vaccination.
- When there was a longer interval between doses, efficacy was higher: 80.7% [95% CI 66.5%-88.9%] at ≥12 weeks vs. 54.9% [95% CI 32.7%-69.7%] at <6 weeks.
Methods: Analysis of combined data for 17,177 participants ≥18 years old through December 7, 2020 from randomized, placebo-controlled phase 3 efficacy trials of ChAdOx1 nCoV-19 against symptomatic COVID-19 in the UK and Brazil, and phase 1/2 clinical trials in the UK and South Africa. Modeled impact of varying the timing of the second dose of vaccine for each 20-day interval. Limitations: Post-hoc data analysis from studies not designed to evaluate efficacy by dosing interval; limited and varying follow-up duration after second dose might bias results; in the UK trial cohort, a subset of participants inadvertently received lower first dose then standard second dose (LD/SD) and were included in the analyses.
Implications: A 3-month dosing interval for the ChAdOx1 nCoV-19 vaccine might optimize the number of people who receive the first dose quickly and reduce disease when supplies are limited. Prospective studies are warranted to confirm durable vaccine efficacy. The longer dosing interval among most participants inadvertently receiving LD/SD might explain the apparent increased efficacy found for this dosing regimen in the interim analysisexternal icon.
Previous SARS-CoV-2 infection might provide immunity to reinfection for an unknown period of time, raising questions about optimal dosing regimen and timing of COVID-19 vaccination in previously infected individuals. Two recent preprints (Krammer et al.external icon, Saadat et al.external icon) report on antibody responses and side effects following COVID-19 vaccination in persons previously infected with SARS-CoV-2.
A. Single dose vaccination in healthcare workers previously infected with SARS-CoV-2.Single dose vaccination in healthcare workers previously infected with SARS-CoV-2.external icon Saadat et al. medRxiv (February 1, 2021). Published in JAMA as Binding and neutralization antibody titers after a single vaccine dose in health care workers previously infected with SARS-CoV-2external icon (March 1, 2021).
Key findings:
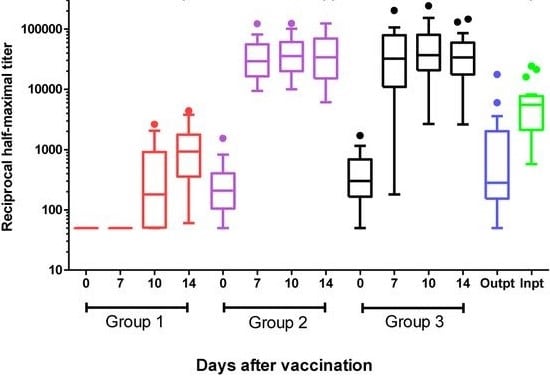
- Healthcare workers (HCW) with prior symptomatic or asymptomatic SARS-CoV-2 infection showed significantly higher antibody binding titers by day 7 after one mRNA vaccine dose compared with vaccinated HCW without prior SARS-CoV-2 infection and compared with unvaccinated COVID-19 patients (Figure).
- At 14 days, HCW with prior SARS-CoV-2 infection who received a single vaccination developed higher neutralization titers than HCW without SARS-CoV-2 infection and SARS-CoV-2 infected patients.
Methods: Measured antibody binding and neutralization titers after a single dose of the BNT162b2 (Pfizer-BioNTech) or mRNA-1273 (Moderna) vaccines in plasma from 59 healthcare workers (HCW) who were either SARS-CoV-2 negative (Group 1), asymptomatic SARS-CoV-2 IgG-positive (Group 2), or symptomatic SARS-CoV-2 IgG-positive (group 3) at 0, 7, 10, and 14 days post-vaccination, and 38 controls (plasma from inpatients and outpatients with COVID-19 at 1 and 2 months after onset of COVID-19 symptoms). Limitations: Unknown how antibody binding and neutralization titers translate to protection from SARS-CoV-2 infection.
Figure.
Note: Adapted from Saadat et al. Anti-SARS-CoV-2 antibody responses after a single vaccination in SARS-CoV-2 IgG-negative healthcare workers (HCW), asymptomatic SARS-CoV-2 IgG-positive HCW, symptomatic SARS-CoV-2 IgG-positive HCW and in unvaccinated COVID-19 outpatients or inpatients. Half-maximal binding titers represent the plasma dilution that achieves 50% of maximal binding of a known control that reaches saturation. Box plots represent the 25th to 75th percentile, with individual dots representing outliers using Tukey’s method (1.5 x IQR). Licensed under CC-BY-NC-ND 4.0
B. Robust spike antibody responses and increased reactogenicity in seropositive individuals after a single dose of SARS-CoV-2 mRNA vaccine.external icon Krammer et al. medRxiv (February 1, 2021). Published in NEJM as Antibody responses in seropositive persons after a single dose of SARS-CoV-2 mRNA vaccineexternal icon (March 10, 2021).
Key findings:
- SARS-CoV-2 seropositive individuals rapidly developed uniform, high median SARS-CoV-2 spike antibody titers after one mRNA vaccine dose that were:
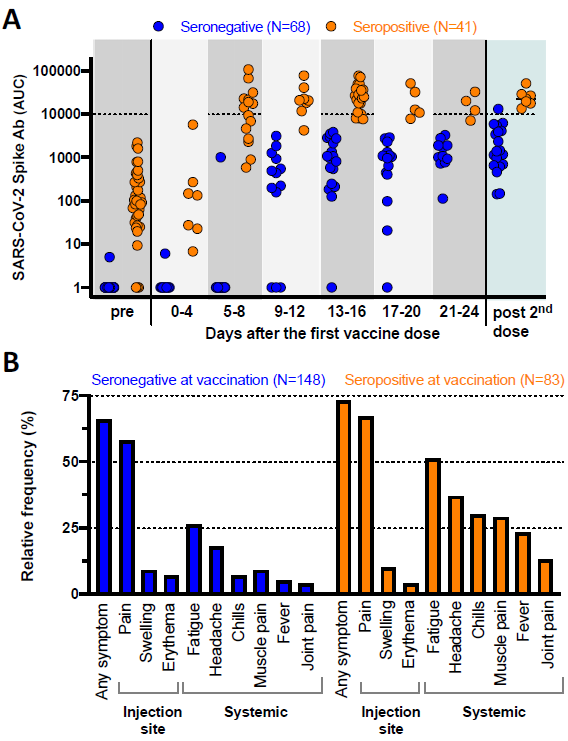
- 10–20 times higher than titers among seronegative individuals after the first mRNA vaccine dose (Figure A).
- More than 10-fold higher than seronegative individuals after the second dose (Figure A).
- Seropositive vaccine recipients experienced systemic side effects (fatigue, headache, chills, fever, muscle or joint pain) more frequently than seronegative vaccine recipients (Figure B).
Methods: Measured antibody responses in 109 individuals who received a first BNT162b2 (Pfizer-BioNTech) or mRNA-1273 (Moderna) vaccine dose in 2020. Compared frequency of reactions after the first dose of vaccination in 231 individuals, which included most of those with antibody results. Limitations: Unknown exactly how antibody titers translate to protection from SARS-CoV-2 infection.
Figure.
Note: Adapted from Krammer et al. A: Quantitative SARS-CoV-2 spike antibody titers (expressed as area under the curve, AUC) for 109 individuals who were seronegative or seropositive at vaccination. “Pre” represents the antibody response prior to vaccination while “post 2nd dose” indicates the immune responses mounted after the second vaccine dose. B: Vaccine-associated side effects experienced after the first dose among 231 individuals who were seronegative or seropositive at vaccination. Licensed under CC-BY-NC-ND 4.0.
Implications for both studies (Krammer et al. and Saadat et al.): One mRNA vaccine dose against SARS-CoV-2 might boost immunity in individuals with a history of SARS-CoV-2 infection; however, additional clinical data are needed to demonstrate protection from reinfection. Limiting vaccination to one dose among seropositive persons could reduce vaccine side effects and extend limited vaccine supply.
PEER-REVIEWED
Assessment of U.S. health care personnel (HCP) attitudes towards COVID-19 vaccination in a large university health care system.external icon Shaw et al. Clinical Infectious Diseases (January 25, 2021).
Key findings:
- Among health care personnel (HCP), intent to vaccinate was higher among men (72.5%) vs. women (52.4%), Asian persons (73.8%) or White persons (58.4%) vs. Black persons (30.8%), and those who did not provide direct patient care (62.4%) vs. those who did (54.0%).
- Of 1,198 registered nurses, 41.2% agreed that they would vaccinate if offered free of charge.
- Adverse events (47%) were most common concern about vaccination.
- Only 16.3% of participants reported that research influences COVID-19 vaccine decision making.
Methods: Cross-sectional analysis of data from 5,287 HCP at SUNY Upstate Medical University, conducted from November 23–December 5, 2020 who completed a validated 22-question electronic survey to ascertain attitudes and beliefs towards vaccination. Limitations: Timing of study one week before the first vaccine emergency use authorization may bias results; ethnicity data were not collected; excluded HCW from nursing homes.
Implications: By identifying concerns about vaccinations and subgroups who are less likely to get vaccinated, these data could inform the vaccine messaging for HCP which might improve vaccine uptake both among HCP and the general public.
Detection, Burden, and Impact
- Lee et al. Impact of comorbid asthma on severity of coronavirus disease (COVID-19)external icon. Scientific Reports (December 11, 2020). In a retrospective cohort study of 7,272 adults with COVID-19, asthma was not a risk factor for poor prognosis, however, among those with asthma [686 (9.4%)] age, male sex, and acute asthma exacerbation in the previous year were significant risk factors for death.
- Karmakar et al. Association of social and demographic factors with COVID-19 incidence and death rates in the US.external icon JAMA Open Network (January 29, 2021). Cross-sectional analyses of 4,289,283 COVID-19 cases and 147,047 deaths from March 25 to July 9, 2020 found that overall social vulnerability index score, racial/ethnic minority status, and limited English proficiency were significantly associated with higher rates of COVID-19 incidence and mortality.
- Karlinsky et al. The World Mortality Dataset: tracking excess mortality across countries during the COVID-19 pandemic.external icon medRxiv (Preprint, January 29, 2021). Published in eLife as Tracking excess mortality across countries during the COVID-19 pandemic with the World Mortality Datasetexternal icon (June 30, 2021). The World Mortality Dataset includes all-cause mortality data from 77 countries used to estimate excess mortality, or an increase in all-cause mortality relative to expected using historical data from 2015-2019, as an objective indicator of COVID-19 deaths.
Natural History of SARS-CoV-2 Infection
- Müller et al. SARS-CoV-2 infects and replicates in cells of the human endocrine and exocrine pancreasexternal icon. Nature Metabolism (February 3, 2021). Although diabetes is a risk factor for severe COVID-19, this study showed that SARS-CoV-2 can directly infect human exocrine and endocrine pancreatic cells causing metabolic dysregulation.
- Amit et al. Post-vaccination COVID-19 among healthcare workers, Israel. Emerging Infectious Diseases (April 2021). Among 4,081 healthcare workers (HCW), 22 (0.54%) had laboratory-confirmed COVID-19 after receiving the first dose of the Pfizer BNT162b2 vaccine; median time to symptom onset after immunization was 3.5 days. See CDC guidance on postvaccine considerations among HCW.
- Surie et al. Infectious period of SARS-CoV-2 in 17 nursing home residents — Arkansas, June–August 2020external icon. Open Forum Infectious Diseases (January 30, 2021). A prospective study of 17 residents at a long term-care facility in Arkansas with median age of 82 years, including one severely immunocompromised person, found that the duration of infectivity was shorter than both duration of symptoms and RT-PCR positivity.
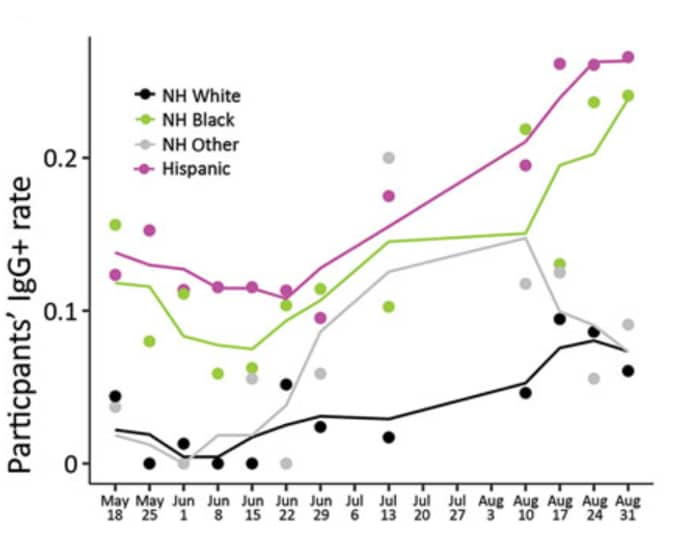
- Letizia et al. SARS-CoV-2 seropositivity among US Marine recruits attending basic training, United States, Spring–Fall 2020. Emerging Infectious Diseases (April 2021). Among 3,196 persons who enrolled in the COVID-19 Health Action Response for Marines Study from May 11 to September 7, 2020, 9% were IgG seropositive; seropositivity was significantly greater among women compared with men, and among Hispanic and non-Hispanic Black persons compared with non-Hispanic White persons (Figure).
Figure.
Note: Adapted from Letizia et al. SARS-CoV-2 IgG-positivity by race and ethnicity: Non-Hispanic (NH) White persons, Non-Hispanic Black persons, Non-Hispanic Other persons, Hispanic persons. Dots indicate weekly IgG-positivity rate for study participants; solid lines indicate 3-week running means. Open access journal; all content freely available.
Prevention, Mitigation, and Intervention Strategies
- Hunter et al. Estimating the effectiveness of the Pfizer COVID-19 BNT162b2 vaccine after a single dose. A reanalysis of a study of ‘real-world’ vaccination outcomes from Israelexternal icon. medRxiv (Preprint, February 3, 2021). Modeling analysis using data from 3,077 persons found that estimated vaccine effectiveness after one dose of the BNT162b2 vaccine was not apparent until after day 14 post-immunization and peaked at 91% on day 21.
- Monod et al. Age groups that sustain resurging COVID-19 epidemics in the United States.external icon Science (February 2, 2021). Model using national-level, aggregate mobility data from 10 million cell phone users in the US found that persons aged 20-49 years are driving the COVID-19 resurgence and should be targeted with prevention interventions.
- Guenezan et al. Povidone iodine mouthwash, gargle, and nasal spray to reduce nasopharyngeal viral load in patients with COVID-19: a randomized clinical trial.external icon JAMA Otolaryngology Head and Neck Surgery (February 4, 2021). ln a randomized clinical trial of 24 persons, nasal decolonization with povidone iodine (PI) was found to be effective at reducing viral titers in the nasopharynx; however, 42% of persons exposed to PI developed thyroid dysfunction which resolved after discontinuation.
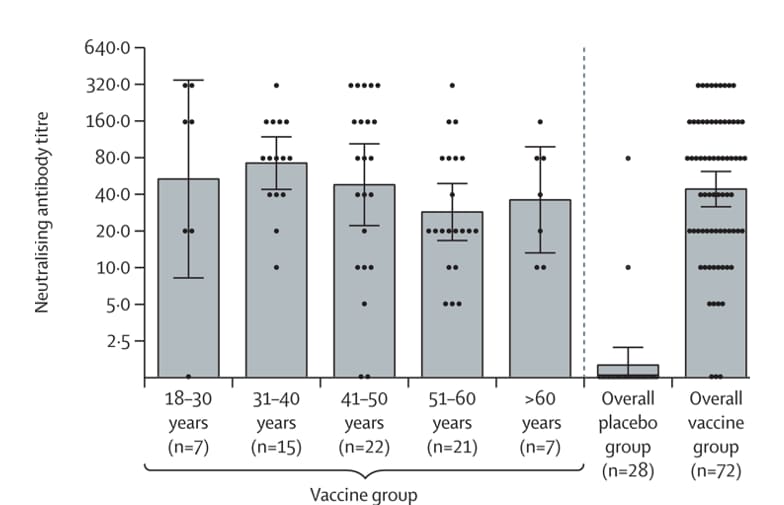
- Logunov et al. Safety and efficacy of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine: an interim analysis of a randomised controlled phase 3 trial in Russiaexternal icon. Lancet (February 2, 2021). Phase 3 randomized controlled trial of 21,977 adults showed a recombinant adenovirus-based vaccine, Gam-COVID-Vac (Sputnik V), has 73.1 % efficacy after the first dose and 91.6% efficacy after the second dose with similar levels of neutralizing titers among men and women and across all age groups.
Figure.
Note: Adapted from Lagunov et al. Neutralizing antibodies on day 42 after first vaccine dose overall and stratified by age compared to placebo group. Dots show individual datapoints, bars show geometric mean titers, and whiskers show 95% CI. Reprinted from The Lancetexternal icon, Copyright (2021), with permission from Elsevier.
Social, Behavioral, and Communication Science
- Ha et al. Using informational murals and handwashing stations to increase access to sanitation among people experiencing homelessness during the COVID-19 pandemicexternal icon. American Journal of Public Health (December 16, 2020). People who experience homelessness are at increased risk of exposure to SARS-CoV-2, thus community partners in Philadelphia installed Wash Your Hands, a series of colorful murals with public health messaging to raise awareness about preventive measures and portable handwashing stations to improve sanitation (Image).
Image.
Note: Adapted from Ha et al. Example of a mural and handwashing station in Philadelphia intending to protect people experiencing homelessness from COVID-19 (Photograph by Conrad Benner). Used by permission of The Sheridan Press.
Disclaimer: The purpose of the CDC COVID-19 Science Update is to share public health articles with public health agencies and departments for informational and educational purposes. Materials listed in this Science Update are selected to provide awareness of relevant public health literature. A material’s inclusion and the material itself provided here in full or in part, does not necessarily represent the views of the U.S. Department of Health and Human Services or the CDC, nor does it necessarily imply endorsement of methods or findings. While much of the COVID-19 literature is open access or otherwise freely available, it is the responsibility of the third-party user to determine whether any intellectual property rights govern the use of materials in this Science Update prior to use or distribution. Findings are based on research available at the time of this publication and may be subject to change.