COVID-19 Science Update released: January 15, 2021 Edition 72

The COVID-19 Science Update summarizes new and emerging scientific data for public health professionals to meet the challenges of this fast-moving pandemic. Weekly, staff from the CDC COVID-19 Response and the CDC Library systematically review literature in the WHO COVID-19 databaseexternal icon, and select publications and preprints for public health priority topics in the CDC Science Agenda for COVID-19 and CDC COVID-19 Response Health Equity Strategy.
Section headings in the COVID-19 Science Update have been changed to align
with the CDC Science Agenda for COVID-19.
Here you can find all previous COVID-19 Science Updates.
Recently reported SARS-CoV-2 variants have raised concerns about effectiveness of current preventive and therapeutic interventions (antibodies and vaccines). Here we report on two studies evaluating effects of mutations in SARS-CoV-2 variants on in vitro antibody neutralization efficacy.
PREPRINTS (NOT PEER-REVIEWED)
A. Comprehensive mapping of mutations to the SARS-CoV-2 receptor-binding domain that affect recognition by polyclonal human serum antibodiesexternal icon. Greaney et al. bioRxiv (January 4, 2021).
Key findings:
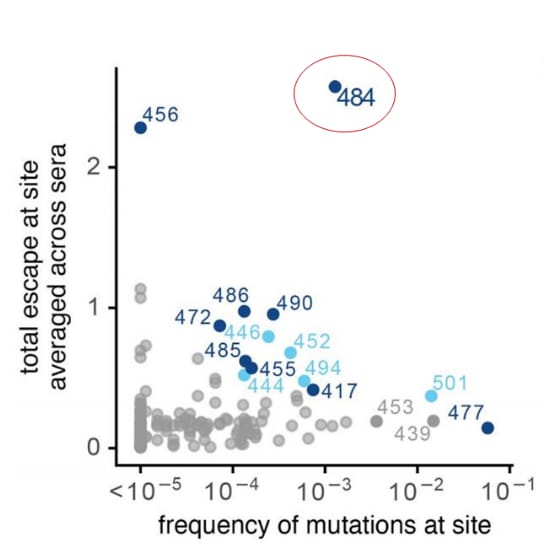
- Mutations at the E484 site of the SARS-CoV-2 spike protein receptor binding protein (RBD) had the largest average effect on neutralizing activity of convalescent polyclonal serum with a >10-fold reduction (Figure).
- E484 mutations were found in 0.11% of sequenced isolates in the Global Initiative on Sharing All Influenza Data (GISAIDexternal icon) library (Figure).
- Impact of mutations on neutralization activity varied markedly across individuals and sometimes in the same individual over time.
Methods: Mapped SARS-CoV-2 spike RBD amino-acid mutations that substantially affected binding by antibodies from 35 plasma samples longitudinally collected from 17 SARS-CoV-2-infected individuals between 15 and 121 days post-symptom onset. Determined frequency of mapped mutations in circulating isolates of SARS-CoV-2 as recorded in GISAIDexternal icon as of December 23, 2020. Limitations: Only examined mutations to the RBD; small donor sample.
Figure:
Note: Adapted from Greaney et al. Effects of mutations at each RBD site (including the receptor-binding ridge and 443-450 loop) on serum antibody binding versus frequency of mutations at each site among all SARS-CoV-2 sequences reported in GISAIDexternal icon as of December 23, 2020. Licensed under CC BY 4.0.
B. SARS-CoV-2 escape in vitro from a highly neutralizing COVID-19 convalescent plasmaexternal icon. Andreano et al. bioRxiv (December 28, 2020).
Key findings:
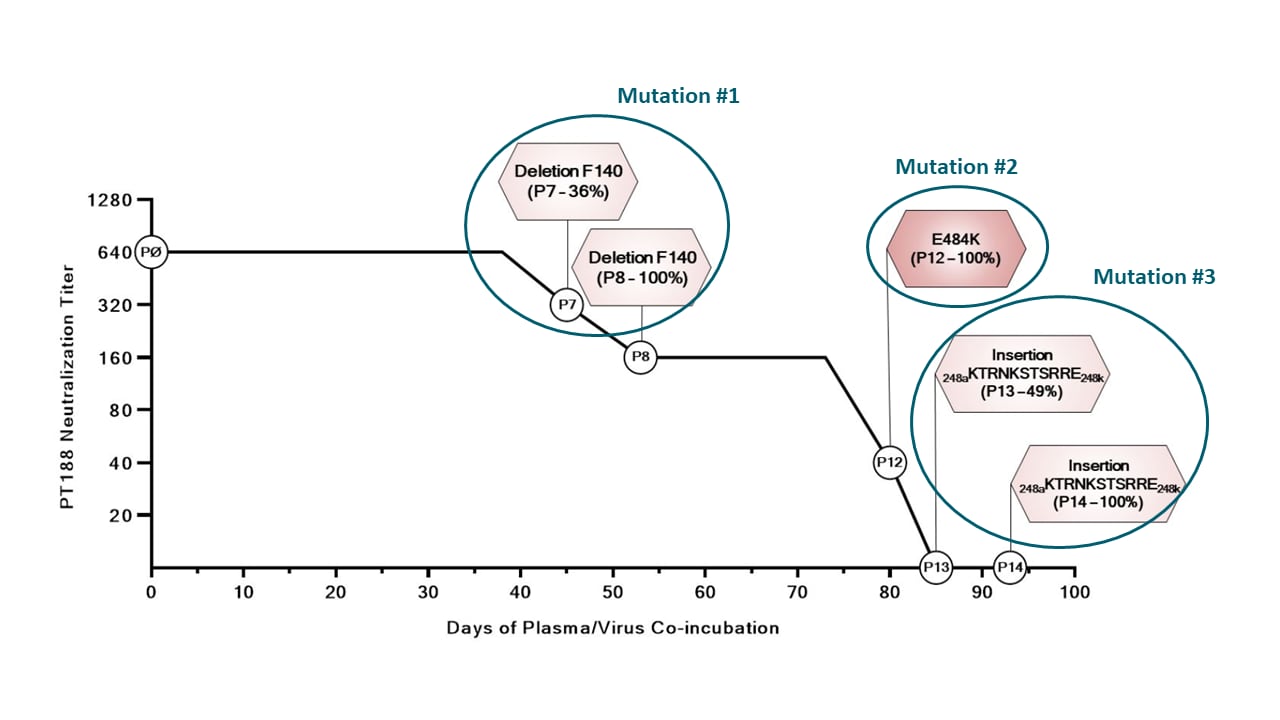
- Convalescent plasma initially neutralized SARS-CoV-2 (titer 1:640), but after multiple passages, neutralization activity declined (titer 1:40) in association with development of 3 viral mutations (Figure):
- A deletion of F140 in the spike N-terminal domain (NTD) N3 loop
- An E484K substitution in the receptor-binding domain (RBD)
- An insertion in the NTD N5 loop
- Convalescent plasma samples and neutralizing monoclonal antibodies showed reduced neutralization activity against variant SARS-CoV-2 virus with 1 or more of the 3 mutations.
Methods: Incubated SARS-CoV-2 virus for more than 90 days with a highly neutralizing plasma from a COVID-19 convalescent patient, selecting a viral escape mutant, and then tested 20 plasma samples from COVID-19 convalescent patients and a panel of 13 neutralizing monoclonal antibodies for neutralization efficacy against the viral variant. Limitations: Single in vitro model of stepwise selection.
Figure:
Note: Adapted from Andreano et al. Timeline of in vitro viral mutations and neutralization titer after each mutation acquired during incubation of SARS-CoV-2 virus with neutralizing plasma from a COVID-19 convalescent patient (PT 188). Specific mutations, incubation passage number, and neutralization titer fold decrease are shown in the hexagons, plotted against days of incubation (x-axis) and neutralization titer (y-axis). Licensed under CC-BY-NC-ND 4.0.
Implications for both studies (Greaney et al. & Andreano et al.): A first case of reinfection with the E484K variant of SARS-CoV-2 was recently reported in Brazil (Nonaka et alexternal icon.). Another recent paper (Kemp et al.external icon) suggests selection pressure on SARS-CoV-2 during convalescent plasma therapy might be associated with emergence of viral variants. Because we are now seeing variants that can evade immune defenses, new monoclonal antibody therapies and possibly new vaccines against emerging variants may be needed. There is an urgent need for widespread uptake of comprehensive prevention measures to reduce SARS-CoV-2 transmission. We should not rely on vaccination alone.
PEER-REVIEWED
Estimation of US SARS-CoV-2 infections, symptomatic infections, hospitalizations, and deaths using seroprevalence surveys.external icon Angulo et al. JAMA Network Open (January 5, 2021).
Key findings:
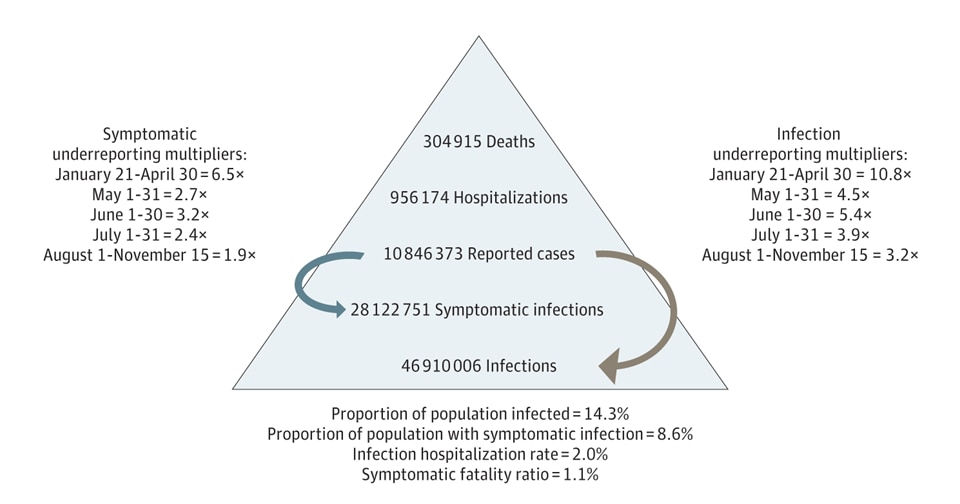
- In the US, compared to 10, 846,373 reported cases, an estimated 46,910,006 SARS-CoV-2 infections occurred through mid-November 2020 (Figure).
- An estimated 3,321,478 infections occurred within the last 7 days of November 15, 2020.
Methods: A cross-sectional study of US participants of all ages was conducted using data collected by CDC from community seroprevalence surveys of the general population (n = 22,118) between April 10 and May 3, 2020 and 4 regional and 1 nationwide seroprevalence surveys between March and August 2021 (n = 95,768). Data were used to estimate infection underreporting and symptomatic underreporting multipliers. Limitations: Derived multipliers are conservative which may lead to underestimation of disease, potential for selection bias, and results may not be nationally representative.
Implications: Seroprevalence data facilitates estimation of true burden of disease as SARS-CoV-2 cases are underreported.
Figure:
Note: Adapted from Angulo et al. COVID-19 burden in the US as of November 15, 2020. Licensed under CC-BY-NC-ND.
Risk factors associated with all-cause 30-day mortality in nursing home residents with COVID-19.external icon Panagiotou et al. JAMA Internal Medicine (January 4, 2021).
Key findings:
- Of 4,127 nursing home residents, 21% died within 30-days of receiving a positive SARS-CoV-2 test result.
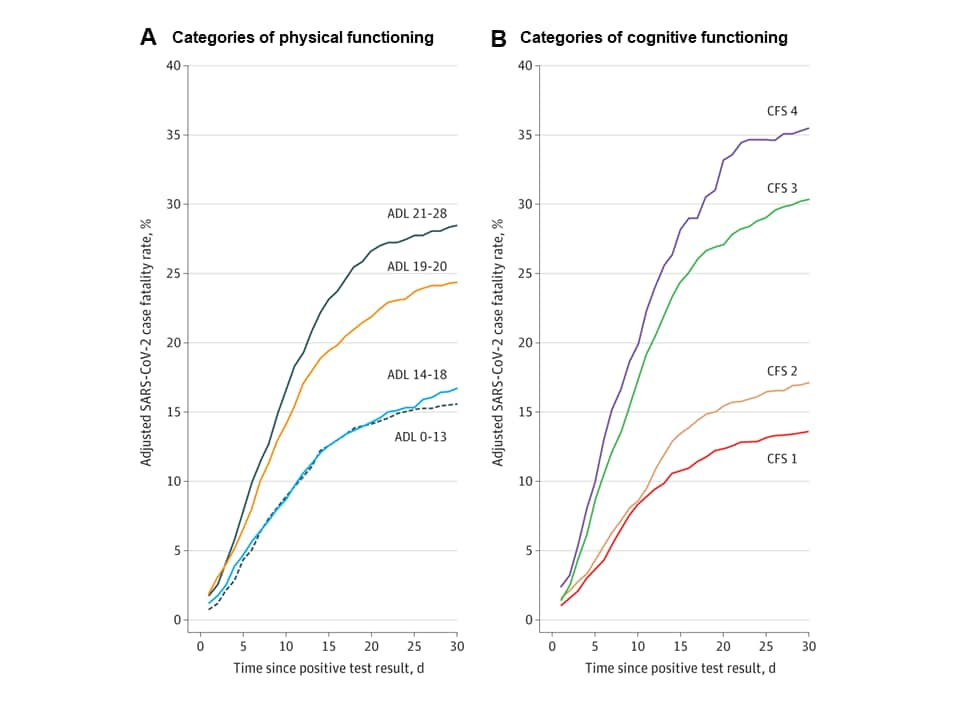
- Physical and cognitive impairments were independently associated with mortality as were diabetes and chronic kidney disease, controlling for age and symptoms (Figure).
Methods: A cohort study of 5,256 nursing home residents with symptomatic, RT-PCR-confirmed SARS-CoV-2 infection from March to September 2020 across 351 facilities in 25 US states assessed risk factors associated with all-cause 30-day mortality. Limitations: Decreased case detection owing to limited test availability; associations might not be causal.
Implications: Physical and cognitive impairments may increase risk for mortality independent from age and comorbidities in this population.
Figure:
Note: Adapted from Panagiotou et al. Case fatality rate by (A) Categories of physical functioning and (B) Categories of cognitive functioning. Physical functioning was assessed with the activities of daily living (ADL) score, with higher values indicating greater impairment. Cognitive functioning was assessed with the Cognitive Function Scale (CFS), which is a hierarchical 4-level scale derived from a resident’s Brief Interview for Mental Status (BIMS) assessment and/or Cognitive Performance Scale (CPS) and integrates their findings into a single score, with higher levels indicating greater impairment. Permission request in process.
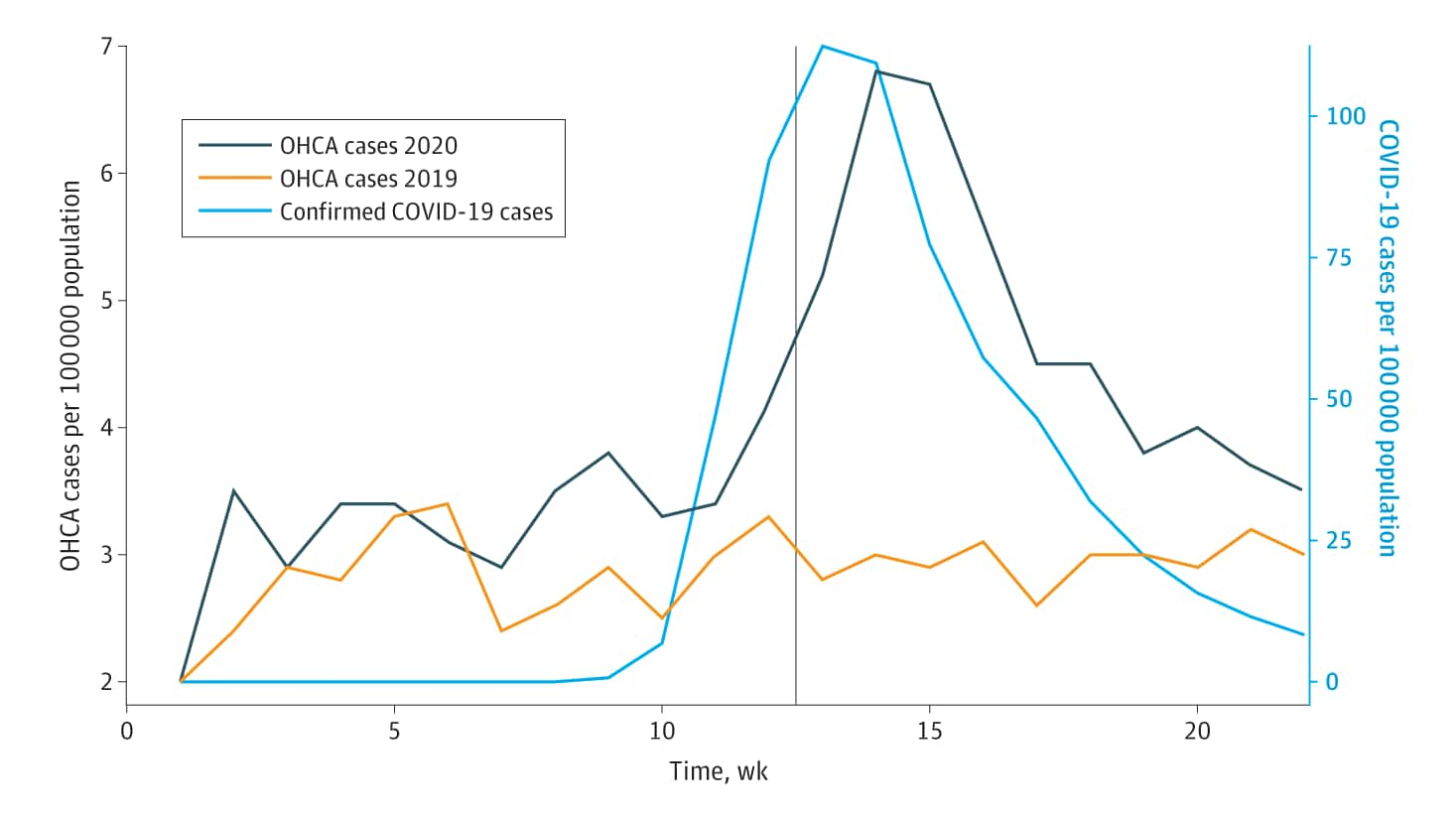
Comparison of out-of-hospital cardiac arrests and fatalities in the metro Detroit area during the COVID-19 pandemic with previous-year events.external icon Nickles et al. JAMA Network Open (January 6, 2021).
Key findings:
- Out-of-hospital cardiac arrest (OHCA) calls increased 60% from 1,162 in 2019 to 1,854 in 2020; in 2020, the peak in the OHCA calls lagged slightly behind the peak of the COVID epidemic curve (Figure).
- OHCA call increases occurred disproportionately among persons over 85 years, Black persons, and residents of nursing facilities.
- A 42% increase in the proportion of OHCA calls for patients who died in the field was noted from 2019 (53.3%) to 2020 (75.5%).
Methods: Weekly per capita incidence of emergency medical services (EMS) calls for OHCA in Detroit were calculated from January 1 to May 31 of both 2019 and 2020 and compared with incident COVID-19 cases. Limitations: Data are limited to pre-admission sources and cannot distinguish between direct effects of SARS-CoV-2 infections or indirect effects of pandemic-related utilization.
Implications: Increased OHCA calls with higher mortality in the field among vulnerable populations warrant further investigations and targeted interventions.
Figure:
Note: From Nickles et al. Weekly incidence of out-of-hospital cardiac arrest calls (left, vertical axis) in metro Detroit from January 1 to May 31 in 2019 and 2020. For comparison, incident COVID-19 cases from the same region are also plotted in light blue and measured by the right, vertical axis. A light grey, vertical line denotes March 23, 2020—the start of Michigan’s stay-at-home order. Licensed under CC-BY.
PEER-REVIEWED
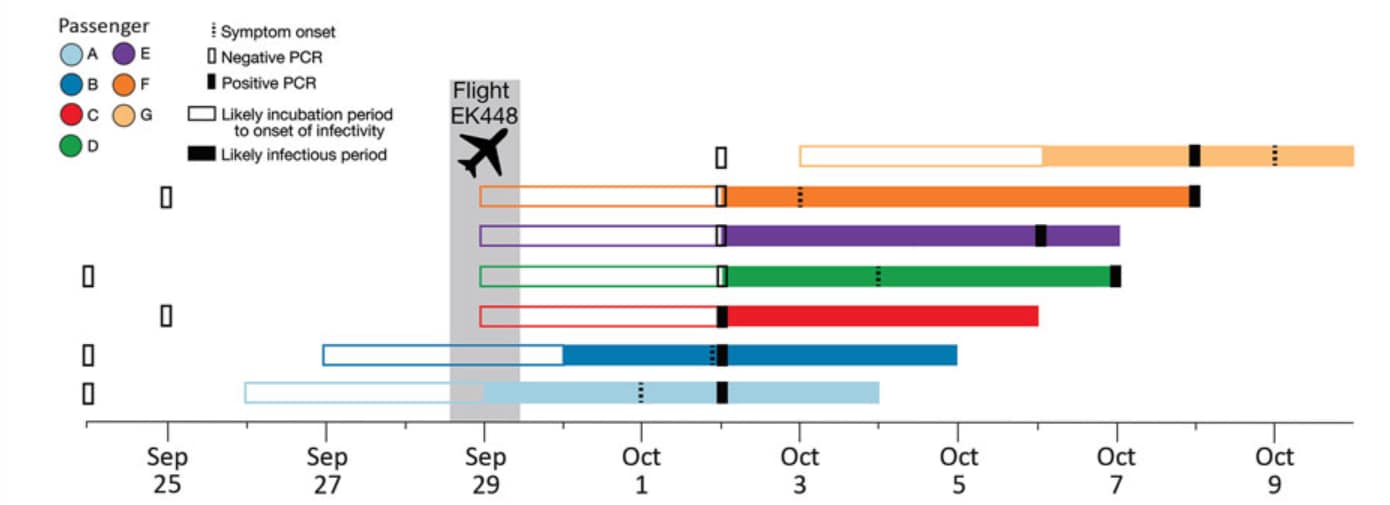
Genomic evidence of in-flight transmission of SARS-CoV-2 despite predeparture testing. Swadi et al. Emerging Infectious Diseases. (January 5, 2021).
Key findings:
- In-flight transmission of SARS-CoV-2 occurred among 7 passengers, 5 of whom tested negative for SARS CoV2 prior to boarding, in aisle seats within a 5-row area of the plane.
- 2 index cases were likely infected before boarding, 4 in-flight, and 1 while in quarantine upon arrival (Figure).
Methods: An investigation was conducted of in-flight transmission using data that included in-flight seating arrangements, symptom onset dates, and genomic data from cases on an 18-hour flight with a 2-hour refueling period that required a 14-day quarantine period at the destination. Limitations: The self-reported preflight negative tests were not described.
Implications: These findings support the need for pre-travel quarantine in addition to a negative test result to prevent in-transit transmission on long-haul flights.
Figure:
Note: Adapted from Swadi et al. Timeline of likely incubation and infectious periods, indicating testing dates, for 7 passengers who tested positive for SARS-CoV-2 infection after traveling on the same flight (EK448) from Dubai, United Arab Emirates, to Auckland, New Zealand, with a refueling stop in Kuala Lumpur, Malaysia, on September 29, 2020. Open access journal; all content freely available.
Rapid transmission of severe acute respiratory syndrome coronavirus 2 in detention facility, Louisiana, USA. Wallace et al. Emerging Infectious Diseases (January 4, 2021).
Key findings:
- From May 7 to June 3, 2020 the cumulative incidence of SARS-CoV-2 infection for all dormitories was 78%.
- Of 111 persons who tested SARS CoV-2 positive, 44% were asymptomatic and 24% reported resolution of symptoms before their first positive test.
- Ct values were similar for symptomatic (range: 19.7–36.3) and asymptomatic persons (19.8–36.9).
- Viral sequences were clustered together and a phylogenetic tree suggested three sequence groups among the different dormitories.
Methods: Prospective cohort investigation at a detention center in Louisiana with SARS CoV-2 testing at several time points of 143 persons in six quarantined dormitories with self-administered questionnaires and medical record abstraction. Limitations: Serial testing was initiated 2–4 weeks after the first case was identified.
Implications: Early initiation of outbreak investigations with serial SARS-CoV-2 testing in congregate facilities, combined with other infection prevention and control efforts may reduce transmission.
PEER-REVIEWED
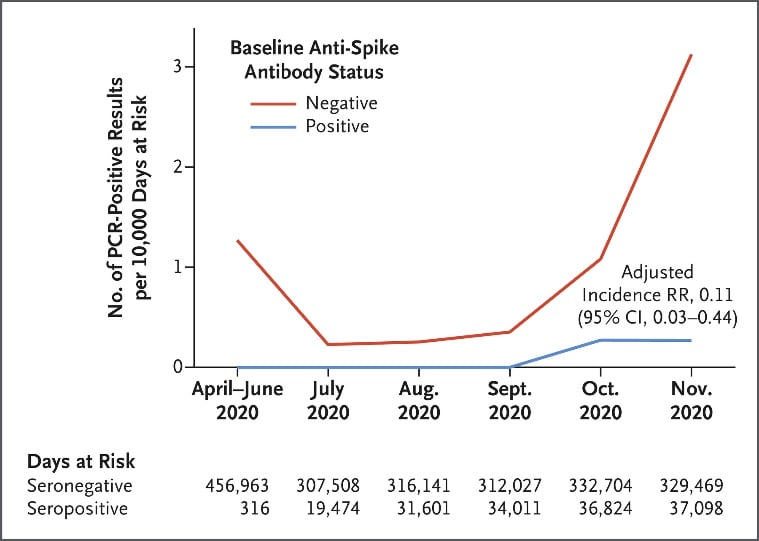
Antibody status and incidence of SARS-CoV-2 infection in healthcare workersexternal icon. Lumley et al. NEJM (December 23, 2020).
Key findings:
- Incidence of SARS-CoV-2 infection was lower among healthcare workers (HCWs) who were anti-spike IgG seropositive (n = 1,265) compared with HCWs who were seronegative (n = 11,364) after up to 31 weeks of follow-up and adjusting for calendar time (Figure).
- Among 3 seropositive HCWs, the time between initial symptoms or seropositivity and reinfection with positive PCR testing ranged from 160 to 199 days.
Methods: Longitudinal cohort study of 12,541 HCWs enrolled at 4 hospitals in the United Kingdom from March 27 to November 30, 2020 investigated the incidence of RT-PCR confirmed SARS-CoV-2 infection according to baseline antibody status. Limitations: Healthy cohort of HCWs <65 years old; short follow-up period; potential for outcome ascertainment bias given less frequent screening among seropositive HCWs.
Implications: Findings suggests that SARS-CoV-2 seropositivity may protect against reinfection for up to 6 months.
Figure:
Note: Adapted from Lumley et al. Observed incidence of SARS-CoV-2 positive PCR results by baseline anti-Spike IgG seronegative and seropositive status among healthcare workers. From NEJM, Lumley et al., Antibody status and incidence of SARS-CoV-2 infection in healthcare workersexternal icon. Copyright © (notice year) Massachusetts Medical Society. Reprinted with permission from Massachusetts Medical Society.
PEER-REVIEWED
Early high-titer plasma therapy to prevent severe COVID-19 in older adultsexternal icon. Libster et al. NEJM (January 6, 2021).
Key findings:
- The risk of developing severe respiratory disease was lower among patients who received convalescent plasma within 72 hours of mild symptom onset compared with patients who received placebo [16% vs 31%, RR = 0.52 (95% CI 0.29-0.94)].
Methods: Randomized, double-blind, placebo-controlled trial of convalescent plasma with high anti-spike protein IgG titers (>1:1,000) against SARS-CoV-2 given within 72 hours onset of mild COVID-19 symptoms, conducted between June 4 and October 25, 2020 among 160 adults aged ≥65 years (80 participants in each group). The primary end point examined in an intent-to-treat analysis was severe respiratory disease. Limitations: Small sample size and lack of statistical power to discern long-term outcomes.
Implications: Early administration of high-titer convalescent plasma against SARS-CoV-2 to mildly ill older adults may reduce progression to severe respiratory disease. Another study showed reduced mortality (Joyner et al.)external icon.
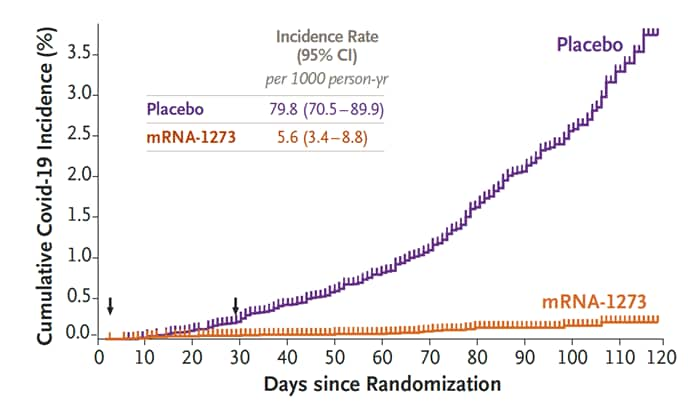
Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine.external icon Baden et al. NEJM (December 30, 2020).
Key findings:
- The mRNA-1273 SARS-CoV-2 vaccine was 94.1% (95% CI 89.3-96.8%) effective (Figure).
- Similar vaccine efficacy was observed in analyses 14 days after the first dose, including participants who had evidence of SARS-CoV-2 infection at baseline, and in participants ≥65 years old.
- 30 cases of severe COVID-19 occurred, with one death, all in the placebo group.
- The incidence of severe adverse events related to treatment was higher among participants receiving the vaccine (0.5%) compared with those receiving the placebo (0.2%).
- Injection-site and systemic adverse events occurred more frequently among the mRNA-1273 group compared with the placebo group and were more common among younger participants aged 18 to 65 years old.
Methods: A phase 3 randomized, observer-blinded, placebo-controlled trial was conducted at 99 centers across the US. Adults aged ≥18 years old at high risk for SARS-CoV-2 infection or complications were randomly assigned 1:1 to receive two intramuscular injections of 100 µg mRNA-1273 (n = 12,134) or placebo (n = 14,073) 28 days apart beginning July 27 to October 23, 2020. Adverse events were monitored and the efficacy of the vaccine in preventing symptomatic COVID-19 illness was assessed. Limitations: Short study duration; no correlate of protection identified; does not assess asymptomatic infection.
Implications: The mRNA-1273 vaccine is safe and effective in preventing symptomatic COVID illness in adults. Findings of fewer cases 14 days after a single dose of mRNA-1273 are promising.
Figure:
Note: Adapted from Baden et al. Efficacy of mRNA-1273 vaccine compared to placebo by cumulative incidence of COVID-19 from time of first dose. Arrows indicate days 1 and 29, when injections were administered. From NEJM, Baden et al., Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccineexternal icon. Copyright © (2020) Massachusetts Medical Society. Reprinted with permission from Massachusetts Medical Society.
Detection, Burden, and Impact
- Nonaka et al. Genomic evidence of a SARS-CoV-2 reinfection case with E484K spike mutation in Brazilexternal icon. Preprints (January 6, 2021). Case report of reinfection with a genetically distinct SARS-CoV-2 variant with an E484K spike mutation associated with increased infectivity and escape from neutralizing antibodies.
- Xie et al. Neutralization of N501Y mutant SARS-CoV-2 by BNT162b2 vaccine-elicited seraexternal icon. bioRxiv (January 7, 2021). Variants containing the N501Y spike mutation did not reduce neutralizing activity with vaccine elicited sera despite affecting the receptor binding domain.
- Kemp et al. Neutralising antibodies in spike mediated SARS-CoV-2 adaptation.external icon medRxiv (December 29, 2020). During convalescent plasma therapy for SARS-CoV-2, strong selection for viral strains with mutations associated with reduced susceptibility to neutralizing antibody can occur.
Transmission
- Johanssen et al. SARS CoV2 transmission from people without COVID-19 symptomsexternal icon. JAMA (January 7, 2021). Modelling estimates that over half of all transmission is attributable to asymptomatic individuals highlighting the need for mitigation measures and strategic testing of these individuals for SARS-CoV-2 control.
Natural History of SARS-CoV-2 Infection: Spectrum and Clinical Course
- Uncini et al. Guillain-Barre syndrome in SARS-CoV-2 infection: An instant systematic review of the first six months of pandemicexternal icon. Neuromuscular Review. (September 15, 2020). Guillain-Barre syndrome, which is responsive to usual treatments, presents as a post-infectious process in persons with SARS-CoV-2 infection suggesting a pathologic link.
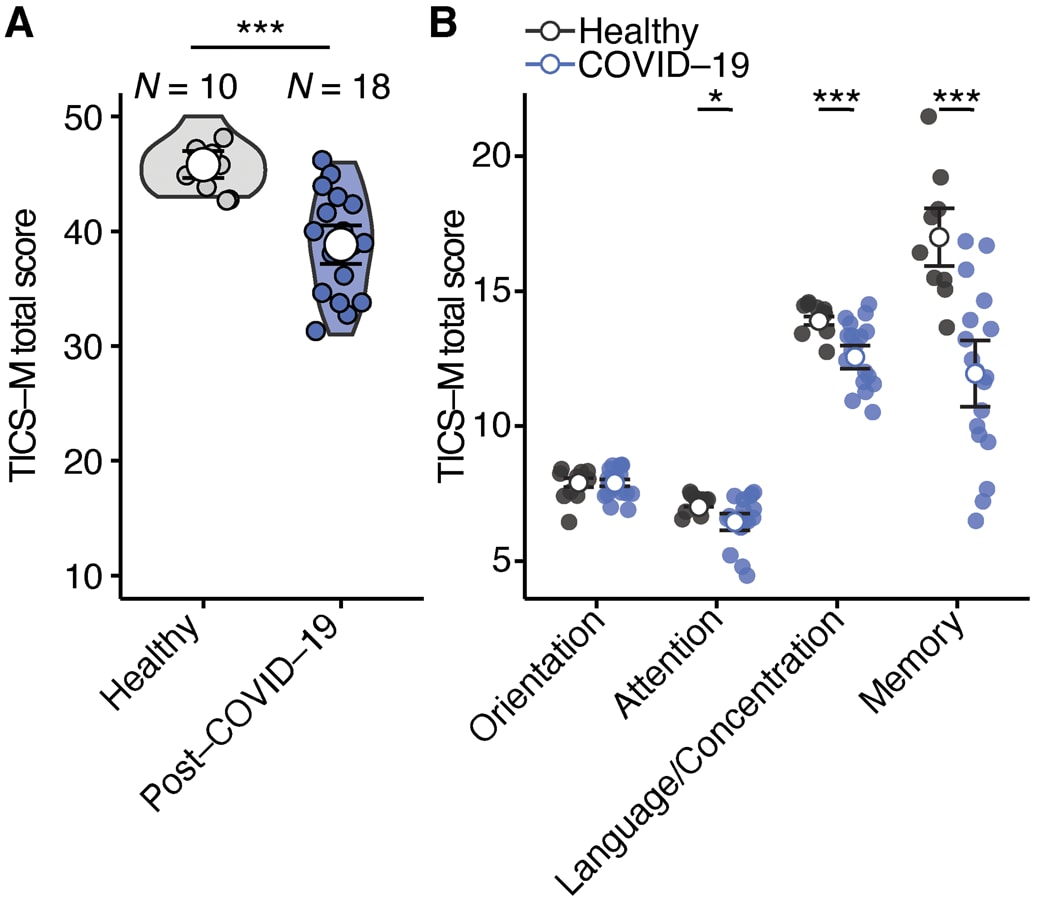
- Woo et al. Frequent neurocognitive deficits after recovery from mild COVID-19.external icon Brain Communications. (November 23, 2020). Among young adults, post-COVID-19 neurocognitive manifestations may occur and require targeted screening for diagnosis (Figure).
Note: Adapted from Woo et al. Cognitive deficiencies in post-COVID-19 patients. (A) Comparison of Modified Telephone Interview for Cognitive Status (TICS-M) total scores (p = 0.0002) between healthy individuals (n = 10) and post-COVID-19 patients (n = 18). Two-tailed Wilcoxon-test was used and mean with 95% confidence interval is shown. (B) Comparison of the different cognitive domains orientation (p = 0.9643), attention (p = 0.029), language and concentration (p = 0.009) and memory (p = 0.004) that were tested with the TICS-M. Two-tailed Wilcoxon-test was used and mean with 95% confidence interval is shown. Licensed under CC-BY-NC.
Prevention, Mitigation, and Intervention Strategies
- Sy et al. Socioeconomic disparities in subway use and COVID-19 outcomes in New York Cityexternal icon. American Journal of Epidemiology (December 29, 2020). Persons who are socially disadvantaged, such as subway users, might not be able to practice certain mitigation measures such as social distancing and thus are at increased risk of SARS-CoV-2 infection.
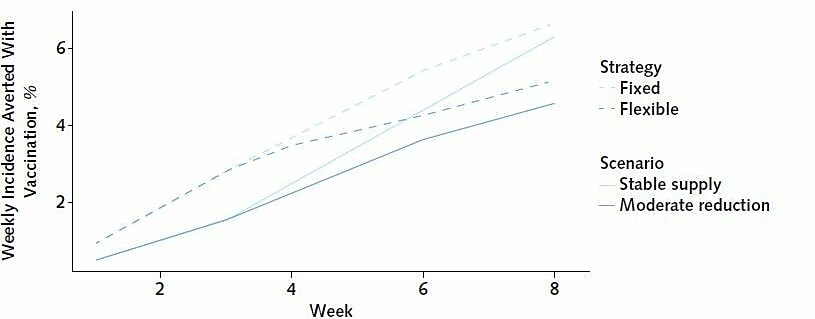
- Tuite et al. Alternative dose allocation strategies to increase benefits from constrained COVID-19 vaccine supply.external icon Annals of Internal Medicine (January 5, 2021). Modeling study implies flexible vaccine dosing strategies can facilitate creating reserves of supplies for the second dose and may result in up to 30% of additional COVID-19 cases averted compared with the fixed strategy (Figure).
Note: Adapted from Tuite et al. Reductions in COVID-19 incidence through the fixed and flexible strategies, under the stable supply scenario and an alternative scenario with reduced supply (down from 6 million doses per week in the first 3 weeks, to 3 million doses per week afterward). Averted incidence expressed as percentage reductions in each week compared with no vaccination, which are not dependent on assumed incidence trends. Permission request in process.
Social, Behavioral, and Communication Science
- Joyner at al. Convalescent plasma antibody levels and the risk of death from COVID-19.external icon NEJM (January 13, 2021). In a retrospective study, early treatment with high titer convalescent plasma was associated with lower risk of death among hospitalized patients with COVID-19 without need for mechanical ventilation.
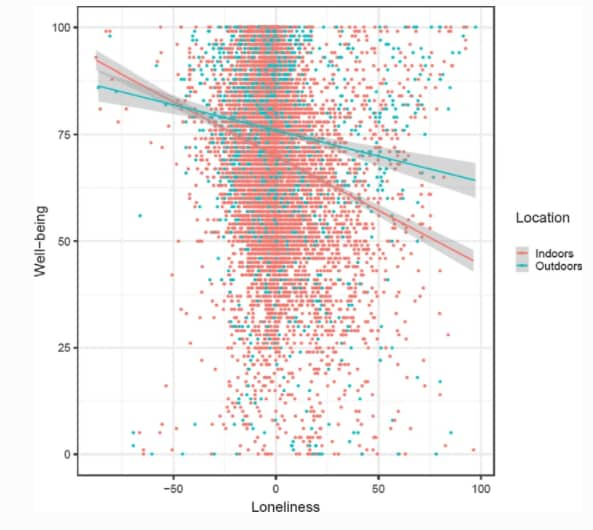
- Stieger et al. Emotional wellbeing under conditions of lockdown: An experience sampling study in Austria during the COVID-19 pandemic.external icon Journal of Happiness Studies (January 2, 2021). In this study, being outdoors was associated with a higher emotional wellbeing and lower impact of loneliness (Figure).
Note: Adapted from Stieger et al. The interaction between loneliness and being outdoors versus indoors on emotional well-being. Loneliness was person-mean centered. Permission request in process.
Disclaimer: The purpose of the CDC COVID-19 Science Update is to share public health articles with public health agencies and departments for informational and educational purposes. Materials listed in this Science Update are selected to provide awareness of relevant public health literature. A material’s inclusion and the material itself provided here in full or in part, does not necessarily represent the views of the U.S. Department of Health and Human Services or the CDC, nor does it necessarily imply endorsement of methods or findings. While much of the COVID-19 literature is open access or otherwise freely available, it is the responsibility of the third-party user to determine whether any intellectual property rights govern the use of materials in this Science Update prior to use or distribution. Findings are based on research available at the time of this publication and may be subject to change.