Acute Flaccid Myelitis Task Force Update
December 5, 2019
Also available as Printable PDF pdf icon[PDF – 178 KB, 10 pages]
Slide 1

Acute Flaccid Myelitis Task Force Update
Co-Chairs: Ruth Lynfield and Tina Tan
Board of Scientific Counselors, Deputy Director for Infectious Diseases
December 5, 2019
Slide 2

AFM Task Force – Terms of Reference
Present findings, observations, and outcomes to CDC Board of Scientific Counselors (BSC) for the Deputy Director for Infectious Diseases for discussion, deliberation, and decision
- Etiologies and pathogenesis
- Evaluate current understanding and the pathogenic mechanisms of AFM
- Review available data and develop hypotheses about possible or likely etiologies and the pathogenesis of AFM
- Propose new studies, study designs, laboratory techniques, assays, and other activities to address specific hypotheses on AFM etiologies and pathogenesis
- Develop and prioritize findings and observations for the BSC to utilize in the development of recommendations for areas of further study or investigation
- Clinical treatment
- Build on existing information on clinical practices by seeking information on clinical experience with the treatment of AFM
- Identify research gaps in the diagnosis and treatment of AFM
- Develop potential findings and observations on patient management
Slide 3

AFM Task Force Membership
BSC Members
- Emily Erbelding, National Institutes of Health, Div. of Microbiology & Infectious Diseases (Ex Officio)
- Ruth Lynfield, Minnesota Dept. of Health
- Tina Tan, Ann & Robert H. Lurie Children’s Hospital of Chicago
AFM Clinical and Research Experts
- Leslie Benson, Boston Children’s Hospital, Dept. of Neurology
- Benjamin Greenberg, University of Texas Southwestern Medical Center, Dept. of Neurology and Neurotherapeutics
- Bryan Grenfell, Princeton University, Ecology and Evolutionary Biology and Public Affairs, Woodrow Wilson School
- Tory Johnson, Johns Hopkins University School of Medicine, Dept. of Neurology
- Bonnie Maldonado, Stanford University School of Medicine, Depts. of Pediatrics and Health Research and Policy
- Kevin Messacar, University of Colorado School of Medicine, Dept. of Pediatrics
- John Modlin, Bill & Melinda Gates Foundation, Polio Research and Policy
- Avi Nath, National Institutes of Health, National Institute of Neurological Disorders and Stroke
- Carlos Pardo-Villamizar, Johns Hopkins University School of Medicine, Dept. of Neurology, Johns Hopkins Transverse Myelitis Center
- Cristina Sadowsky, Kennedy Krieger Institute, International Center for Spinal Cord
- Matthew Schniederjan, Emory University School of Medicine, Dept. of Pathology
- Nate Smith, Arkansas Dept. of Health
- Jill Taylor, New York State Dept. of Health, Wadsworth Laboratory
- Ken Tyler, University of Colorado School of Medicine, Chair, Dept. of Neurology
- Arun Venkatesan, Johns Hopkins University School of Medicine, Div. and Physical Medic of Neuroimmunology and Neuroinfectious Diseases
Slide 4

AFM Task Force – Activities Since May 2019 BSC Meeting
- May, June, September, October 2019: conference calls
- Discussion of outcome measures
- Updated CSTE case definition
- CDC communications including AFM Vital Signs
- Lab updates
- Epidemiology Updates
- Update on the NIH natural history study
- Transverse Myelitis Association/Siegel Rare Neuroimmune Association
- November 2019: in-person meeting
- Reviewed CDC preparedness activities for 2020 season
- Reviewed ongoing research projects and other activities
- Prioritized research questions
Slide 5

AFM Task Force – Main Categories for Action and Research
- Communication and education
- Surveillance
- Diagnostic tool development
- Pathogenesis
- Risk factors studies
- Therapeutics and vaccines
- Treatment and rehabilitation
Slide 6
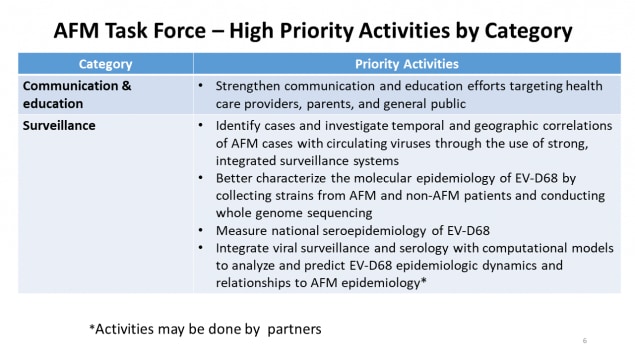
AFM Task Force – High Priority Activities by Category
| Category | Priority Activities |
|---|---|
| Communication & education |
|
| Surveillance |
|
*Activities may be done by partners
Slide 7
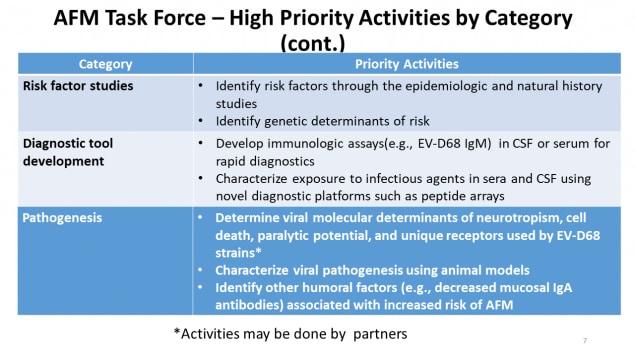
AFM Task Force – High Priority Activities by Category (continued)
| Category | Priority Activities |
|---|---|
| Risk factor studies |
|
| Diagnostic tool development |
|
| Pathogenesis |
|
*Activities may be done by partners
Slide 8
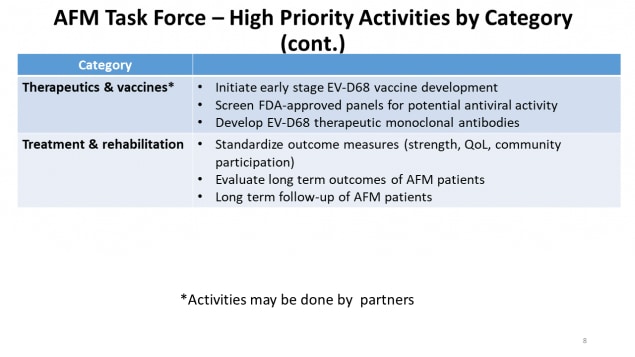
AFM Task Force – High Priority Activities by Category (continued)
| Category | Priority Activities |
|---|---|
| Therapeutics & vaccines* |
|
| Treatment & rehabilitation |
|
*Activities may be done by partners
Slide 9

Acknowledgements
AFM Task Force Members, Sarah Kidd, Manisha Patel, Janell Routh, Adriana Lopez, Mona Marin, Angela Guo, Amie Nisler, Jeanette St. Pierre, Tom Clark, Steve Oberste, Mark Pallansch, William Weldon, Jennifer Anstadt, Allan Nix, Nancy Messonnier
Slide 10

Questions for BSC
- Preparedness
- Any other considerations for CDC’s response plan?
- Does BSC endorse the response plan?
- Research agenda
- Additional suggestions to make the CDC research agenda more robust?
- Priorities
- Any other high priority areas for consideration?
- Other recommendations?