Ciprofloxacin for Post-Exposure Prophylaxis of Anthrax
Emergency Use Instructions for Healthcare Providers
This fact sheet provides instructions for the use of ciprofloxacin for post-exposure prophylaxis (PEP) during an emergency involving anthrax (referred to as Emergency Use Instructions (EUI) fact sheet). Ciprofloxacin is FDA-approved for PEP of inhalation anthrax – to reduce the incidence or progression of disease following exposure to aerosolized Bacillus anthracis (B. anthracis).1 The Food and Drug Administration (FDA) has also issued an order permitting the emergency dispensing of oral formulations of ciprofloxacin without a prescription during an anthrax emergency to individuals who may have been exposed to B. anthracis.2
What is inhalation anthrax?
Anthrax is a serious disease caused by the spore-forming bacterium B. anthracis. Inhalation anthrax is the most deadly form of the disease, with a historical mortality rate of approximately 90% for untreated cases. Inhalation anthrax occurs when an individual inhales aerosolized spores. It is not spread from person to person. Early symptoms include fever, chills, fatigue, cough or headache. Later symptoms include shortness of breath, chest pain, confusion or nausea. Symptoms usually occur within 7 days of inhaling anthrax spores, but can occur as soon as 24 hours after exposure or may take up to 6 to 7 weeks to appear (animal data show symptoms can occur more than 50 days after exposure).
Who should NOT take ciprofloxacin?
Do not give ciprofloxacin to anyone who is allergic to a quinolone antibiotic (including ciprofloxacin) or has a history of myasthenia gravis. Avoid concomitant administration of ciprofloxacin and Zanaflex (tizanidine) since ciprofloxacin can increase effects of tizanidine (e.g., bradycardia, hypotension); consider switching either ciprofloxacin or tizanidine to an alternative drug.
What is the usual dose of ciprofloxacin for PEP of anthrax?
The full PEP regimen is 60 days. During an anthrax emergency, recipients may receive an initial 10-day supply to begin ciprofloxacin therapy; public health officials will announce whether recipients need more ciprofloxacin and how to get additional quantities of the drug.
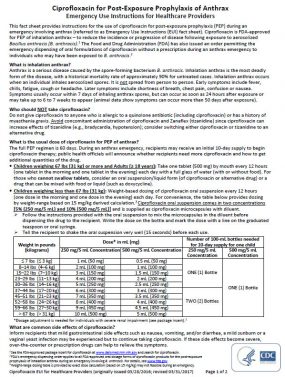
- Children weighing 67 lbs (31 kg) or more and Adults (≥ 18 years): Take one tablet (500 mg) by mouth every 12 hours (one tablet in the morning and one tablet in the evening) each day with a full glass of water (with or without food). For those who cannot swallow tablets, consider an oral suspension/liquid form (of ciprofloxacin or alternative drug) or a drug that can be mixed with food or liquid (such as doxycycline).
- Children weighing less than 67 lbs (31 kg): Weight-based dosing of ciprofloxacin oral suspension every 12 hours (one dose in the morning and one dose in the evening) each day. For convenience, the table below provides dosing by weight-range based on 15 mg/kg derived calculation.3 Ciprofloxacin oral suspension comes in two concentrations [5% (250 mg/5 mL) and 10% (500 mg/5 mL)] and is supplied as ciprofloxacin microcapsules with diluent.
- Follow the instructions provided with the oral suspension to mix the microcapsules in the diluent before dispensing the drug to the recipient. Write the dose on the bottle and mark the dose with a line on the graduated teaspoon or oral syringe.
- Tell the recipient to shake the oral suspension very well (15 seconds) before each use.
Ciprofloxacin Oral Suspension: 250 mg/5 mL Concentration
| Weight in pounds (kilograms) | Dose* in mL (mg) | Number of 100-mL bottles needed for 10-day supply for one child |
|---|---|---|
| ≤ 7 lbs (≤ 3 kg) | 1 mL (50 mg) | ONE (1) Bottle |
| 8–14 lbs (4–6 kg) | 2 mL (100 mg) | ONE (1) Bottle |
| 15–22 lbs (7–10 kg) | 3 mL (150 mg) | ONE (1) Bottle |
| 23–29 lbs (11–13 kg) | 4 mL (200 mg) | ONE (1) Bottle |
| 30–36 lbs (14–16 kg) | 5 mL (250 mg) | ONE (1) Bottle |
| 37–44 lbs (17–20 kg) | 6 mL (300 mg) | TWO (2) Bottles |
| 45–51 lbs (21–23 kg) | 7 mL (350 mg) | TWO (2) Bottles |
| 52–58 lbs (24–26 kg) | 8 mL (400 mg) | TWO (2) Bottles |
| 59–66 lbs (27–30 kg) | 9 mL (450 mg) | TWO (2) Bottles |
| > 67 lbs (> 31 kg) | 10 mL (500 mg) | TWO (2) Bottles |
*Dosage adjustment is needed for individuals with severe renal impairment (see package insert).1
Ciprofloxacin Oral Suspension: 500 mg/5 mL Concentration
| Weight in pounds (kilograms) | Dose* in mL (mg) | Number of 100-mL bottles needed for 10-day supply for one child |
|---|---|---|
| ≤ 7 lbs (≤ 3 kg) | 0.5 mL (50 mg) | ONE (1) Bottle |
| 8–14 lbs (4–6 kg) | 1 mL (100 mg) | ONE (1) Bottle |
| 15–22 lbs (7–10 kg) | 1.5 mL (150 mg) | ONE (1) Bottle |
| 23–29 lbs (11–13 kg) | 2 mL (200 mg) | ONE (1) Bottle |
| 30–36 lbs (14–16 kg) | 2.5 mL (250 mg) | ONE (1) Bottle |
| 37–44 lbs (17–20 kg) | 3 mL (300 mg) | ONE (1) Bottle |
| 45–51 lbs (21–23 kg) | 3.5 mL (350 mg) | ONE (1) Bottle |
| 52–58 lbs (24–26 kg) | 4 mL (400 mg) | ONE (1) Bottle |
| 59–66 lbs (27–30 kg) | 4.5 mL (450 mg) | ONE (1) Bottle |
| > 67 lbs (> 31 kg) | 5 mL (500 mg) | ONE (1) Bottle |
*Dosage adjustment is needed for individuals with severe renal impairment (see package insert).1
What are common side effects of ciprofloxacin?
Inform recipients that mild gastrointestinal side effects such as nausea, vomiting, and/or diarrhea, a mild sunburn or a
vaginal yeast infection may be experienced but to continue taking ciprofloxacin. If these side effects become severe,
over-the-counter or prescription drugs can help to relieve the symptoms.
What are possible side effects of ciprofloxacin?
Tell recipients to STOP the ciprofloxacin and get medical help immediately if they develop any of the following:
- Tendon rupture, tendinitis or joint problems
- Serious allergic/hypersensitivity reactions (anaphylactic and/or severe rashes)
- Liver problems (anorexia, jaundice, dark brown or tea-colored urine, pruritus or tender abdomen)
- Central nervous system effects (seizures, tremors, paranoia, anxiety)
- Serious heart rhythm changes (QT prolongation and torsade de pointes)
- Severe stomach cramps with high fever or bloody diarrhea (antibiotic-associated diarrhea and pseudomembranous colitis)
- Changes in sensation and possible nerve damage (peripheral neuropathy)
What should recipients avoid while taking ciprofloxacin?
- If a recipient is taking Carafate (sucralfate), Videx (didanosine), phosphate binders or multivitamins, supplements or antacids containing magnesium, calcium, aluminum, iron or zinc, instruct the recipient to take ciprofloxacin at least 2 hours before or 6 hours after taking any of these products.
- Ciprofloxacin can interact with certain drugs such as blood thinners (increased blood thinning), oral antidiabetic drugs (increased glucose-lowering effect), phenytoin (loss of seizure control), theophylline (increased theophylline concentration), or clozapine (irregular heartbeat). If a recipient is on these or other drugs with known interaction with ciprofloxacin, consider changing the dose of these drugs or recommending alternative drugs. For more information on ciprofloxacin drug interactions, please see package insert.
What additional information should be provided to recipients taking ciprofloxacin?
- Ciprofloxacin can exacerbate myasthenia gravis symptoms. It can also greatly potentiate effects of Zanaflex (tizanidine) (e.g., bradycardia, hypotension). Instruct those with a history of myasthenia gravis or taking tizanidine to avoid taking ciprofloxacin.
- Instruct recipients not to take ciprofloxacin with dairy products (like milk or yogurt) or calcium-fortified juices.
- Ciprofloxacin can cause sun sensitivity. Instruct recipients to use sunscreen and cover exposed skin.
- Ciprofloxacin, while not generally recommended for use in pregnancy, is recommended as antimicrobial PEP for anthrax during pregnancy and while breastfeeding due to the risks of anthrax. The very limited data available on ciprofloxacin use in pregnancy suggest the benefits of ciprofloxacin outweigh the risks.
- Recipients may wish to cut back on their caffeine intake, as the caffeine half-life may be prolonged.
- Instruct recipients to keep ciprofloxacin tablets dry and to store tablets and reconstituted oral suspension at room temperature (68–77°F or 20–25°C). Reconstituted oral suspension may be stored at room temperature up to 14 days.
- If you have been asked to dispense ciprofloxacin with an expired date on the container, please note that FDA is allowing for the use of certain lots of ciprofloxacin beyond the labeled expiration date during an anthrax emergency based on scientific review. For more information, go to the FDA website at www.fda.govexternal icon (search for “ciprofloxacin expiration”).
- The Countermeasures Injury Compensation Program (CICP) is a federal program created to help pay for related costs of medical care and other specific expenses for eligible people seriously injured by the administration or use of certain medical countermeasures. Medical countermeasures may include vaccines, medications, devices or other items used to prevent, diagnose or treat the public during a public health emergency or security threat. For more information about CICP, visit www.hrsa.gov/cicpexternal icon or call: 1-855-266-2427.
Risk‐Benefit Statement
Although ciprofloxacin has some potential and serious adverse events, the expected benefit of ciprofloxacin to help prevent disease and death associated with anthrax exposure outweigh these risks.
Available Alternatives
During an anthrax emergency, you will be informed of any alternative antibiotics that are available, such as doxycycline, levofloxacin or amoxicillin. The risks and benefits of available alternative antibiotics will be explained in their own fact sheets.
Reporting Adverse Event or Medication Errors
Report adverse events or medication errors to MedWatchexternal icon by completing a MedWatch Form 3500 or by calling 1‐800‐FDA‐1088.
1 See the FDA‐approved package insert for ciprofloxacin at www.dailymed.nlm.nih.govexternal icon and search for ciprofloxacin.
2 FDA’s emergency dispensing order applies to all FDA-approved oral dosage forms of ciprofloxacin products for the post-exposure
prophylaxis of inhalation anthrax during an emergency involving B. anthracis. For details, see www.fda.govexternal icon.
3 Weight-range dosing table is provided as exact dose calculation (based on 15 mg/kg) may not be feasible during an emergency.
NOTE: Ciprofloxacin EUI for Healthcare Providers (originally issued 03/28/2016; revised 08/18/2017)