 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
| ||||||||||
|
|
|
|
|
Persons using assistive technology might not be able to fully access information in this file. For assistance, please send e-mail to: mmwrq@cdc.gov. Type 508 Accommodation and the title of the report in the subject line of e-mail. Guidelines for Using the QuantiFERON®-TB Gold Test for Detecting Mycobacterium tuberculosis Infection, United StatesAn erratum has been published for this article. To view the erratum, please click here. Prepared by
The material in this report originated in the National Center for HIV, STD, and TB Prevention, Kevin Fenton, MD, PhD, Director, and the Division of Tuberculosis Elimination, Kenneth G. Castro, MD, Director. Corresponding address: CDC/National Center for HIV, STD, and TB Prevention/Division of Tuberculosis Elimination; 1600 Clifton Road, NE, MS E-10, Atlanta, GA 30333. Telephone: 404-639-8120; Fax: 404-639-8604; E-mail: mai9@cdc.gov. Summary On May 2, 2005, a new in vitro test, QuantiFERON®-TB Gold (QFT-G, Cellestis Limited, Carnegie, Victoria, Australia), received final approval from the U.S. Food and Drug Administration as an aid for diagnosing Mycobacterium tuberculosis infection. This test detects the release of interferon-gamma (IFN-g) in fresh heparinized whole blood from sensitized persons when it is incubated with mixtures of synthetic peptides representing two proteins present in M. tuberculosis: early secretory antigenic target--6 (ESAT-6) and culture filtrate protein--10 (CFP-10). These antigens impart greater specificity than is possible with tests using purified protein derivative as the tuberculosis (TB) antigen. In direct comparisons, the sensitivity of QFT-G was statistically similar to that of the tuberculin skin test (TST) for detecting infection in persons with untreated culture-confirmed tuberculosis (TB). The performance of QFT-G in certain populations targeted by TB control programs in the United States for finding latent TB infection is under study. Its ability to predict who eventually will have TB disease has not been determined, and years of observational study of substantial populations would be needed to acquire this information. In July 2005, CDC convened a meeting of consultants and researchers with expertise in the field to review scientific evidence and clinical experience with QFT-G. On the basis of this review and discussion, CDC recommends that QFT-G may be used in all circumstances in which the TST is currently used, including contact investigations, evaluation of recent immigrants, and sequential-testing surveillance programs for infection control (e.g., those for health-care workers). This report provides specific cautions for interpreting negative QFT-G results in persons from selected populations. This report is aimed at public health officials, health-care providers, and laboratory workers with responsibility for TB control activities in the United States. BackgroundOn May 2, 2005, a new in vitro test, QuantiFERON®-TB Gold (QFT-G, manufactured by Cellestis Limited, Carnegie, Victoria, Australia), received final approval from the U.S. Food and Drug Administration (FDA) as an aid in diagnosing Mycobacterium tuberculosis infection, including both latent tuberculosis infection (LTBI) and tuberculosis (TB) disease. This enzyme-linked immunosorbent assay (ELISA) test detects the release of interferon-gamma (IFN-g) in fresh heparinized whole blood from sensitized persons when it is incubated with mixtures of synthetic peptides simulating two proteins present in M. tuberculosis: early secretory antigenic target--6 (ESAT-6) and culture filtrate protein--10 (CFP-10). ESAT-6 and CFP-10 are secreted by all M. tuberculosis and pathogenic M. bovis strains. Because these proteins are absent from all Bacille Calmette-Guérin (BCG) vaccine strains and from commonly encountered nontuberculous mycobacteria (NTM) except M. kansasii, M. szulgai, and M. marinum (1), QFT-G is expected to be more specific for M. tuberculosis than tests that use tuberculin purified protein derivative (PPD) as the antigen. QFT-G represents one type of IFN-g release assay (IGRA) (2). Tests such as QFT-G measure the IFN-g released by sensitized white blood cells after whole blood is incubated with antigen. Tests such as ELISpot enumerate cells releasing IFN-g after mononuclear cells recovered from whole blood are incubated with similar antigens. Two IGRAs have been approved by FDA for use in the United States: the original QuantiFERON®-TB test (QFT) and the recently approved QFT-G. The two tests use different antigens to stimulate IFN-g release, different methods of measurement, and different approaches to test interpretation. QFT was approved as an aid for diagnosing LTBI, whereas QFT-G is approved as an aid for diagnosing both LTBI and TB disease. QFT is no longer commercially available. Before QFT was approved in 2001, the tuberculin skin test (TST) was the only test available for detecting LTBI (3). QFT-G is intended to replace QFT. QFT-G results can be available <24 hours after testing without the need for a second visit, whereas a TST requires a second encounter to read the result 48--72 hours after administration of the test. As a laboratory-based assay, QFT-G is not subject to biases and errors of TST placement and reading. However, errors in collecting or transporting blood specimens or in running and interpreting the assay can decrease the accuracy of QFT-G. Related to the uncertainty in interpreting a test result, including that of the TST, when the test's measurement approaches a fixed cut-off point, the reproducibility of QFT-G is less when the measured amount of IFN-g is near the test's cut-off point. Detection of substantial amounts of released IFN-g in the nil sample disallows arriving at a negative test result. Each of the three tests (TST, QFT, and QFT-G) relies on a different immune response and differs in its relative measures of sensitivity and specificity. The TST assesses in vivo delayed-type hypersensitivity (Type IV), whereas QFT and QFT-G measure in vitro release of IFN-g. The TST and QFT measure response to PPD, a polyvalent antigenic mixture, whereas QFT-G measures response to a mixture of synthetic peptides simulating two specific antigenic proteins that are present in PPD. The agreement between TST and QFT results in persons at increased risk for LTBI facilitated approval and acceptance of QFT (3,4). Results of similar studies using QFT-G testing for persons at increased risk have not been published, but less agreement between TST and QFT-G results is predictable because fewer and more specific antigens are used in QFT-G. QFT-G is not affected by prior BCG vaccination (1) and is expected to be less influenced by previous infection with nontuberculous mycobacteria (5). TSTs are variably affected by these factors. QFT-G does not trigger an anamnestic response (i.e., boosting) because it does not expose persons to antigen. Injection of PPD for the TST can boost subsequent TST responses, primarily in persons who have been infected with NTM or vaccinated with BCG. Compared with the TST, QFT-G might be less affected by boosting from a previous TST. Assessment of the accuracy of QFT-G and other indirect tests for M. tuberculosis infection (including TSTs) is hampered by the lack of confirmatory tests to diagnose LTBI and culture-negative TB disease (6). This lack is partially addressed by observing the proportion of negative tests among persons who are unlikely to have M. tuberculosis infection because they lack risks (this approach approximates specificity); by observing the proportion positive among persons with culture-confirmed TB disease (this approach approximates sensitivity); and by determining factors associated with discordance between a new test and the TST. One limitation of the first approach is that certain persons who have no recognized risks might be infected with M. tuberculosis, which causes specificity to be underestimated. A broad limitation is that the TST and any newer tests might not perform the same for detecting LTBI as they do for detecting M. tuberculosis infection during TB disease. For example, reduction of in vitro IFN-g release has been attributed to suppressive cytokines associated with TB disease (7). When comparing an IGRA with a TST, variations in methods also must be considered (e.g., use of different antigens or risk-stratified cut-off points for interpreting results). Studies assessing QFT-G with these approximation methods have been published (5,8,9). A specificity of 98.1% was reported in 216 BCG-vaccinated Japanese nursing students who were entering their training and who were at low risk for M. tuberculosis infection, and a sensitivity of 89.0% was reported in 118 patients with culture-confirmed TB (5). However, QFT-G results were derived slightly differently than the methods approved by FDA. In another study (8), QFT-G was compared with TST by using two tuberculin units of RT-23 (8,10). In a group of 99 healthy, BCG-vaccinated medical students in Korea, the specificity of QFT-G was 96%, compared with 49% for the TST. Among 54 patients with pulmonary TB disease, the sensitivity of the QFT-G was 81%, compared with 78% for the TST (8). QFT-G and the TST were compared in an unselected population of 318 hospitalized patients (9). QFT-G had greater sensitivity for TB disease (67%) than did TST (33%), but indeterminate QFT-G responses were common (21%) among patients with negative TST results, the majority of whom were thought to be immunocompromised or immunosuppressed. The antigens or laboratory methods in other studies have varied (2). Although the findings are informative, how QFT-G will perform in the same circumstances is unknown. In an investigation of contacts in a high school in Denmark in which a student had infectious TB, the same ELISA used with QFT-G was employed, but with recombinant ESAT-6 and CFP-10 antigens used rather than the mixtures of synthetic peptides used with QFT-G (11). The IGRA used in that study agreed well with the TST in non-BCG--vaccinated contacts. BCG-vaccinated contacts were not skin tested, but their IGRA results closely paralleled those for the nonvaccinated contacts, which suggested that BCG vaccination was not affecting the results of this IGRA. MethodologyDuring July 11--12, 2005, CDC convened a meeting in Atlanta, Georgia, of consultants and researchers with expertise in the field to review studies and assess experience with QFT-G. Unpublished data from studies of QFT-G were considered in preparing these guidelines. Expert consultants (see Membership List), researchers, TB control public health practitioners, and representatives of FDA, other federal agencies, and the manufacturer reviewed the evolving data on QFT-G. Data from ongoing studies evaluating QFT-G in U.S. Navy recruits, correctional facility inmates, persons with suspected TB disease, contacts of persons suspected to have TB disease, and health care workers were reviewed. For developing these guidelines, CDC considered the scientific evidence and the opinions of the consultants. Their opinions did not represent endorsement from their organizations. This report provides interim guidance for use and interpretation of QFT-G. Confirming or excluding TB disease and assessing the probability of LTBI require a combination of epidemiologic, historic, physical, and diagnostic findings that should be considered when interpreting QFT-G results. This report is intended to assist public health officials, clinicians, and laboratorians in their efforts to understand the use of QFT-G for TB control. Indications for QFT-GFDA approved QFT-G as an in vitro diagnostic aid using peptide mixtures simulating ESAT-6 and CFP-10 proteins to stimulate cells in heparinized whole blood. Detection of IFN-g by ELISA is used to identify in vitro responses to ESAT-6 and CFP-10 that are associated with M. tuberculosis infection (12). From a medical and public health perspective, QFT-G testing is indicated for diagnosing infection with M. tuberculosis, including both TB disease and LTBI. Whenever M. tuberculosis infection or disease is being diagnosed by any method, the optimal approach includes coordination with the local or regional public health TB control program. How QFT-G Testing is Performed and InterpretedInstructions for the QFT-G assay are in the package insert (13). Aliquots of heparinized whole blood are incubated with the test antigens for 16--24 hours. The blood must be incubated with the test antigens <12 hours after collection. Test kits include two mixtures of synthetic peptides representing ESAT-6 and CFP-10 as test antigens, phytohemaglutinin (a mitogen used as a positive assay control), and saline (used as a nil sample to measure the background level of IFN-g). After incubation, the concentration of IFN-g in the plasma is determined by ELISA by using the reagents included in the test kit. The amount of IFN-g released is determined by subtracting the amount in the nil from the amount in the ESAT-6, CFP-10, or mitogen-stimulated plasma. QFT-G test results can be calculated by using software provided by the manufacturer. This report provides guidelines for interpreting test results (Table). Laboratory reports should include interpretation of QFT-G test results and indicate the concentration of IFN-g in each plasma sample. Cautions and LimitationsCertain limitations of QFT-G are similar to those of the TST, but these limitations have not been studied extensively for QFT-G. Whereas the sensitivity of QFT-G for detecting M. tuberculosis infection in persons with untreated culture-confirmed TB is approximately 80% in published studies (5,8), its sensitivity for particular groups of TB patients (e.g., young children and immunocompromised patients) has not been determined. QFT-G sensitivity for LTBI might be less than that of the TST, although the lack a confirmatory test makes this difficult to assess. Estimating the sensitivity of any indirect test for LTBI by testing patients who have TB disease might be inaccurate because of differences between these conditions. The ability of QFT-G to predict risk for LTBI progressing subsequently to TB disease has not been determined. QFT-G, as with the TST, cannot differentiate infection associated with TB disease from LTBI. A diagnosis of LTBI requires that TB disease be excluded by medical evaluation, which should include checking for suggestive symptoms and signs, a chest radiograph, and, when indicated, examination of sputum or other clinical samples for the presence of M. tuberculosis. Similar to any other diagnostic test, the predictive value of QFT-G results depends on the prevalence of M. tuberculosis infection in the population being tested. Each QFT-G result and its interpretation should be considered in conjunction with other epidemiologic, historic, physical, and diagnostic findings. As with a negative TST result, negative QFT-G results should not be used alone to exclude M. tuberculosis infection in persons with symptoms or signs suggestive of TB disease. The presence of symptoms or signs suggestive of TB disease increases the likelihood that M. tuberculosis infection is present, and these circumstances decrease the predictive value of a negative QFT-G or TST result. Medical evaluation of such persons should include a history and physical examination, chest radiograph, bacteriologic studies, serology for human immunodeficiency virus (HIV), and, when indicated, other tests or studies. The performance of QFT-G, in particular its sensitivity and its rate of indeterminate results, has not been determined in persons who, because of impaired immune function, are at increased risk for M. tuberculosis infection progressing to TB disease. Impaired immune function can be caused by HIV infection or acquired immunodeficiency syndrome (AIDS); current treatment with immunosuppressive drugs including high-dose corticosteroids, tumor necrosis factor-alpha (TNF-a) antagonists, and drugs used for managing organ transplantation; selected hematologic disorders (e.g., myeloproliferative disorders, leukemias, and lymphomas); specific malignancies (e.g., carcinoma of the head, neck, or lung); diabetes; silicosis; and chronic renal failure (6). Each of these conditions or treatments is known or suspected to decrease responsiveness to the TST, and they also might decrease production of IFN-g in the QFT-G assay. Consequently, as with a negative TST result, negative QFT-G results alone might not be sufficient to exclude M. tuberculosis infection in these persons. Published data are relatively limited concerning the use of QFT-G among persons recently exposed to TB (e.g., contacts) and other populations at high risk for LTBI. No published data document the performance of QFT-G in children aged <17 years. With any of the testing methods, persons who have a negative test result can still have LTBI. Those who have a negative result but who are likely to have LTBI and who are at greater risk for severe illness or poor outcomes if TB disease occurs might need treatment or closer monitoring for disease (6). Potential examples include close contacts who are aged <5 years, those who are immunocompromised because of HIV infection, or those who will undergo treatment with TNF-a antagonists (which increase the risk for progression from LTBI to TB disease) (14--16). QFT-G has practical limitations that include the need to draw blood and to ensure its receipt in a qualified laboratory in time for testing. The blood must be incubated with the test antigens <12 hours after collection, while the lymphocytes are viable. After the blood is incubated with antigens for 16--24 hours, plasma must be collected and either properly stored or tested promptly by ELISA. Collecting the required 5-mL blood sample from younger children might not be possible or acceptable. Additional Considerations and Recommendations in the Use of QFT-G in Testing ProgramsQFT-G can be used in all circumstances in which the TST is used, including contact investigations, evaluation of recent immigrants who have had BCG vaccination, and TB screening of health-care workers and others undergoing serial evaluation for M. tuberculosis infection. QFT-G usually can be used in place of (and not in addition to) the TST. A positive QFT-G result should prompt the same public health and medical interventions as a positive TST result. No reason exists to follow a positive QFT-G result with a TST. Persons who have a positive QFT-G result, regardless of symptoms or signs, should be evaluated for TB disease before LTBI is diagnosed. At a minimum, a chest radiograph should be examined for abnormalities consistent with TB disease. Additional medical evaluation would depend on clinical judgment on the basis of findings from history (including exposure to infectious TB), physical examination, and chest radiography. HIV counseling, testing, and referral is recommended because HIV infection increases the suspicion for TB and the urgency of treating LTBI. After TB has been excluded, treatment of LTBI should be considered (6). The majority of healthy adults who have negative QFT-G results are unlikely to have M. tuberculosis infection and do not require further evaluation. However, for persons with recent contact with persons who have infectious TB, negative QFT-G results should be confirmed with a repeat test performed 8--10 weeks after the end of exposure, as is recommended for a negative TST result. Studies to determine the best time to retest contacts with negative QFT-G results have not been reported. Until more information is available, the timing of QFT-G testing should be the same as that used for the TST (17,18). When "window period" prophylaxis (i.e., treatment for presumed LTBI) is indicated for contacts aged <5 years or severely immunocompromised persons who are exposed to highly contagious TB, repeat testing for LTBI is recommended 8--10 weeks after contact has ended (18). With either TST or QFT-G, negative results of the test at the end of the window period should be interpreted by considering all available epidemiologic, historic, clinical, physical, and diagnostic information, including the findings for the other contacts in the investigation. A full course of treatment should be considered even with a negative result from either test at the end of the window period when the rate of M. tuberculosis transmission to other contacts was high or when a false-negative result is suspected because of a medical condition (18). A greater rate of positive results has been reported with TST than with QFT-G in persons with and without recognized risks for M. tuberculosis infection, except for patients who have culture-confirmed TB disease (5,8). This tendency might be explained by either greater specificity with QFT-G, greater sensitivity with TST, or both. For this reason, all information must be considered when making treatment decisions for persons with increased risk for progression from LTBI to TB or in whom TB disease is associated with increased risk for severe illness or poor outcomes. An indeterminate QFT-G result does not provide useful information regarding the likelihood of M. tuberculosis infection. The optimal follow-up of persons with indeterminate QFT-G results has not been determined. The options are to repeat QFT-G with a newly obtained blood specimen, administer a TST, or do neither. For persons with an increased likelihood of M. tuberculosis infection who have an indeterminate QFT-G result, administration of a second test, either QFT-G or TST, might be prudent. The potential for TST to cause boosting and the need for two-step testing in settings conducting serial testing should be considered. For persons who are unlikely to have M. tuberculosis infection, no further tests are necessary after an indeterminate QFT-G result. Laboratories should report the reason that the QFT-G result was indeterminate (e.g., high background levels of IFN-g in the nil sample or inadequate response to mitogen). In one report, inadequate response to mitogen was associated with immunosuppressive conditions (9). As with the TST, if TB disease is suspected, additional diagnostic evaluations should be performed before or at the same time as the QFT-G and should not be delayed while awaiting QFT-G results. These evaluations should include chest radiography, bacteriologic studies, serology for HIV, and, as indicated by the illness, additional tests and studies. At present, as with the TST, the results of indirect tests for M. tuberculosis (e.g., QFT-G) usually would not influence the selection of additional tests and studies in such patients. TB control programs can use QFT-G for investigating contacts of persons with potentially infectious TB disease. Because QFT-G does not require a second visit to complete, test results probably will be available from a greater percentage of contacts than would be available using TST. Because of its greater specificity, QFT-G is expected to indicate a smaller proportion of contacts as infected than the TST would indicate. Public health resources that previously were devoted to completion of testing can instead be concentrated on full evaluation and complete treatment of contacts who have positive QFT-G results. In contrast to the TST, initial QFT-G testing of contacts will not boost subsequent test results, which avoids uncertainty about interpreting follow-up results. However, QFT-G might be less sensitive for LTBI than the TST, and its ability to predict subsequent development of TB disease is undetermined. QFT-G might represent a cost-effective alternative to the TST in testing programs which are part of the TB infection control program in institutions such as health care settings, correctional facilities, or homeless shelters. In these settings, false-positive reactions to the TST pose a problem. This problem is compounded in settings with BCG-vaccinated persons born in countries where TB is prevalent. Follow-up visits for reading the TST also pose substantial operational challenges; the second visit for reading requires extra effort and leads to inefficiency. The greater specificity of the QFT-G and the requirement for only one visit are compelling advantages. General recommendations on the use of QFT-G as part of the infection control program in health-care settings have been included in the most recent revision of the TB infection control guidelines (19). In situations with serial testing for M. tuberculosis infection, initial two-step testing, which is necessary with the TST, is unnecessary with QFT-G and is not recommended. TB control programs or institutions that elect to use QFT-G should consult and collaborate with laboratories in their system to ensure that specimens are properly obtained, handled, and processed prior to and after arrival in the laboratory. Information concerning the assay is in the package insert (13). Training of laboratory staff will be necessary. Certain facilities might elect to refer specimens for testing. The Clinical Laboratory Improvement Amendments (CLIA) regulations for quality systems of all phases of the total testing process (pre-analytic, analytic, and post-analytic) and for general laboratory systems must be followed, including, but not limited to, the requirements for test system, equipment, instruments, reagents, materials and supplies (42 CFR Part 493.1252), and the establishment or verification of performance specifications (42 CFR Part 493.1253) (20). In addition, under CLIA, documentation of all quality systems, including laboratory proficiency and staff competency, is required. Future Research NeedsAdditional studies to assess the performance of the QFT-G test under program conditions should be conducted. Further research is needed regarding use of QFT-G in multiple clinical circumstances. Studies of test performance should assess specificity, sensitivity, reproducibility, and association of test results with risk for infection and risk for progressing to TB disease. Comparisons among different IGRAs and TSTs are encouraged. Questions to be addressed include the following:
In collaboration with FDA and the manufacturer, CDC will establish mechanisms for postmarketing surveillance. Providers should use FDA's MedWatch (available at http://www.accessdata.fda.gov/scripts/medwatch ) to report instances of a contact having all of the following criteria:
Certain instances consistent with these criteria might require further study of the circumstances. However, reliance on postmarketing surveillance is not a substitute for research targeted at the above-noted questions. Research in these areas and others should therefore be conducted through prospective studies. The optimal methods for ensuring quality in laboratory implementation of QFT-G testing should be determined. Educational materials are needed that can be widely disseminated to educate physicians regarding the use of the QFT-G assay. CDC will work with partners and the manufacturer to ensure the development of such materials. Other IGRA tests and test formats might become available in the United States over the next several years (21,22). Users of any of these products should anticipate the need for periodic modifications in practice, with resulting improvements in utility of these testing technologies. Acknowledgments The following persons and groups provided vital assistance in the preparation of this report: Sandra Monique Arend, MD, PhD, Leiden University Medical Center, Leiden, The Netherlands; Antonino Catanzaro, MD, University of California San Diego and Cellestis, San Diego, California; Raymond Chinn, MD, Hospital Infection Control Professional Advisory Committee representative to the Advisory Committee for the Elimination of Tuberculosis, Atlanta, Georgia; Charles L. Daley, MD, Michael D. Iseman, MD, National Jewish Medical and Research Center, Denver, Colorado; Kimberly Field, MSN, Washington State Department of Health, Tumwater, Washington and the National Tuberculosis Controllers Association; Nobuyuki Harada, PhD, Kazue Higuchi, PhD, Research Institute of Tuberculosis, Japan Anti-Tuberculosis Association, Tokyo, Japan; C. Robert Horsburg, MD, Boston University School of Public Health, Boston, Massachusetts; Madhukar Pai, MD, PhD, University of California, Berkeley and San Francisco, California; Luca Richeldi, MD, PhD, University of Modena and Reggio Emilia, Modena, Italy; Jason E. Stout, MD, Duke University, Durham, North Carolina; Michael Tapper, MD, Lenox Hill Hospital, New York, New York; Paul Vinton, MBBS, Royal Melbourne Hospital and Monash Medical Centre, Melbourne, Australia; David Warshauer, PhD, Association of Public Health Laboratories, Washington, District of Columbia; Tony Radford, PhD, James Rothel, PhD, Mark Boyle, Cellestis Limited, Carnegie, Australia; Steve M. Ostroff, MD, Department of Health and Human Services, Honolulu, Hawaii; In vitro Diagnostic Device Evaluation and Safety, Center for Devices and Radiological Health, Food and Drug Administration, Washington, District of Columbia; Adelisa L. Panlilio, MD, Division of Health Care Quality Promotion, National Center for Infectious Diseases; David N. Weisman, MD, Division of Respiratory Disease Studies, National Institute for Occupational Safety and Health; Division of Laboratory Systems, National Center for Health Marketing; Terrence L. Chorba, MD, Ronald O. Valdiserri, MD, National Center for HIV, STD, and TB Elimination; Division of Tuberculosis Elimination, National Center for HIV, STD, and TB Prevention, CDC, Atlanta, Georgia. References
CDC Expert Consultation on
QuantiFERON®-TB Gold
Chair: Neil Schluger, MD, Columbia University, New York City, New York. Members: John Bernardo, MD, Boston University School of Medicine, Boston, Massachusetts; Henry Blumberg, MD, PhD, Emory University School of Medicine, Atlanta, Georgia; Nancy Warren, PhD, Association of Public Health Laboratories, Washington, DC; Masae Kawamura, MD, San Francisco Department of Public Health, San Francisco, California; David Lewinsohn, MD, PhD, Oregon Health and Science University, Portland, Oregon; Edward Nardell, MD, Harvard School of Public Health, Cambridge, Massachusetts; Tanya Oemig, National Tuberculosis Controllers' Association, Smyrna, Georgia; Randall Reves, MD, Denver Public Health Department, Denver, Colorado; Stephen Kralovic, MD, Veterans Administration, Cincinnati, Ohio; Rachel Stricof, MPH, Association for Professionals in Infection Control and Epidemiology, Albany, New York; Gail Woods, MD, University of Arkansas for Medical Sciences, Little Rock, Arkansas. Table 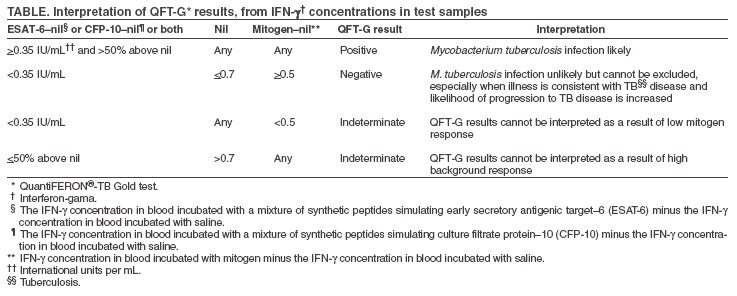 Return to top.
All MMWR HTML versions of articles are electronic conversions from ASCII text into HTML. This conversion may have resulted in character translation or format errors in the HTML version. Users should not rely on this HTML document, but are referred to the electronic PDF version and/or the original MMWR paper copy for the official text, figures, and tables. An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S. Government Printing Office (GPO), Washington, DC 20402-9371; telephone: (202) 512-1800. Contact GPO for current prices. **Questions or messages regarding errors in formatting should be addressed to mmwrq@cdc.gov.Date last reviewed: 12/9/2005 |
|||||||||
|