Clinical Immunization Safety Assessment (CISA) Project
Vaccine Safety Monitoring - CISA
CDC’s Clinical Immunization Safety Assessment (CISA) Project was established in 2001 to address the unmet vaccine safety clinical research needs of the United States.
CISA is a national collaborating network of vaccine safety experts from the CDC’s Immunization Safety Office (ISO), eight medical research centers, and other partners. CDC established the CISA Project to improve the understanding of adverse events following immunization at the individual patient level. CISA provides consultations for U.S. healthcare providers with complex vaccine safety questions about their patients and conducts vaccine safety clinical research.
Healthcare providers or health departments in the United States can request a consultation from CISA COVIDvax for a complex COVID-19 vaccine safety question that is (1) about an individual patient residing in the United States or vaccine safety issue and (2) not readily addressed by CDC or Advisory Committee on Immunization Practices (ACIP) guidelines.
This request can be made through CDC-INFO by:
- Calling 800-CDC-INFO (800-232-4636), or
- Submitting a request via CDC-INFO webform
When making the request, indicate that the request is for CISA Project consultation and it will be forwarded to the CISA COVIDvax clinicians for review.
Provide the following in the form:
- Your name and phone number
- Your health professional training category (i.e., medical doctor, nurse practitioner, pharmacist)
- Indication that a CISA COVIDvax consultation is requested
- Enough clinical background information to enable CDC CISA clinical staff to evaluate the inquiry properly.
Do not include patient names or personal identifying health information in the request.
In case of an emergency clinical COVID-19 vaccine safety inquiry, healthcare providers and health department staff can call the CDC Emergency Operations Center (EOC) Watch Desk at (770)-488-7100. The EOC Watch Desk will route emergency inquiries to the CISA COVIDvax on-call staff.

If you are a U.S. healthcare provider with a vaccine safety question unrelated to COVID-19 vaccines about a specific patient residing in the U.S., you can contact CISA at CISAeval@cdc.gov to request a case evaluation. This service is provided free of charge. View here for more information.
In case of an emergency clinical vaccine safety inquiry, healthcare providers and health department staff can call the CDC Emergency Operations Center (EOC) Watch Desk at (770)-488-7100. The EOC Watch Desk will route emergency inquiries to the on-call staff.
Current CISA Project Sites
Collaboration between CDC and eight medical research centers
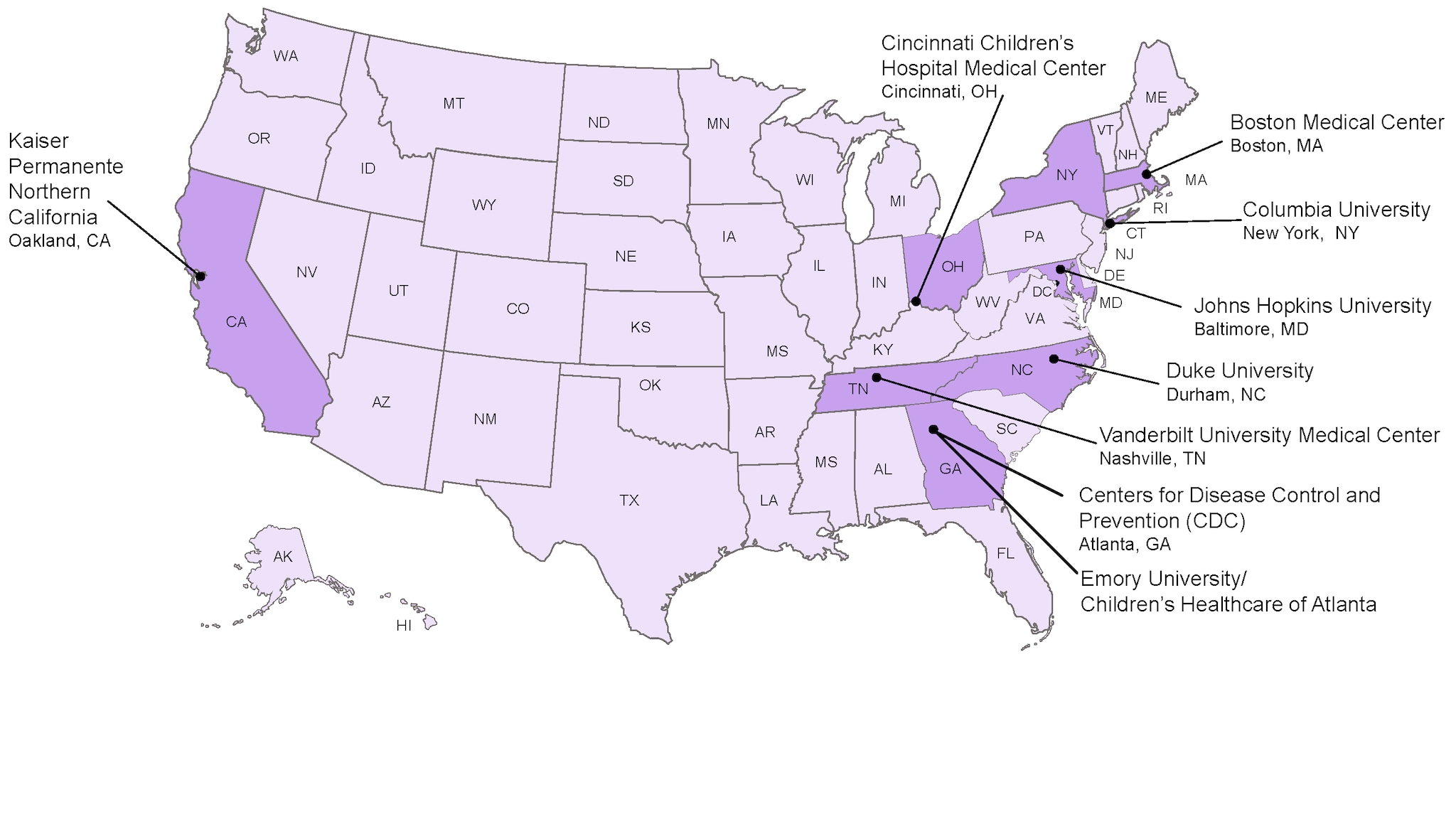
The eight medical research centers that are involved in the CISA network include:
- Boston Medical Center Boston, MA
- Cincinnati Children’s Hospital Medical Center Cincinnati, OH
- Columbia University New York, NY
- Duke University Durham, NC
- Emory University/Children’s Healthcare of Atlanta, Atlanta, GA
- Johns Hopkins University Baltimore, MD
- Kaiser Permanente Northern California Oakland, CA
- Vanderbilt University Medical Center Nashville, TN
About CISA
The goals of CISA are to:
- Serve as a vaccine safety resource for U.S. healthcare providers with complex vaccine safety questions about a specific patient to assist with immunization decision-making
- Assist CDC and its partners in evaluating emerging vaccine safety issues
- Conduct clinical research studies to better understand vaccine safety and identify preventive strategies for adverse events following immunization
CISA Current Activities
Clinical Case Reviews: CISA provides a clinical case consultation service for U.S. healthcare providers who have vaccine safety questions about a specific patient residing in the United States. CISA provides clinical expertise in various disciplines, including infectious diseases, neurology, allergy, immunology, pediatrics, hematology, and obstetrics/gynecology.
Expert Evaluation of Vaccine Safety Issues: CISA experts provide advice that has led to a broader understanding of vaccine safety issues . CISA has also contributed to clinical guidance and Advisory Committee on Immunization Practices (ACIP) recommendations pertinent to vaccine safety.
Research: To advance knowledge of vaccine safety and inform clinical and public health practice clinical research is essential. CISA has published and continues to develop research studies that address vaccine safety questions.
Current priority areas for CISA research studies include COVID-19 and influenza vaccine safety, and vaccine safety in pregnant women. CISA complements other vaccine safety systems and focuses its efforts on scalable, prospective studies for U.S.-licensed or authorized vaccines. These studies are designed to address clinical vaccine safety questions in targeted or special populations that are often excluded from pre-licensure clinical trials. CISA is well suited to study more common, non-medically attended events (e.g., fever) and to collect biological specimens after vaccination. CISA investigators also have access to special populations (e.g., pregnant women) and specialists who care for these patients.
Public Health Response: CISA has procedures in place to assist in response to vaccine safety emergencies, such as a pandemic, and to assist state health departments during an emergency. Since December 2020, CISA has provided consultation to U.S. healthcare providers and health departments about COVID-19 vaccine safety. Request a COVID-19 CISA Clinical Consultations.